A Practical Guide to Fluorescent Labeling of Antibodies: Principles, Challenges, and Applications
Antibody labeling, as a core technology in modern biological research and diagnostic experiments, enables highly sensitive detection and visualization of target proteins by coupling specific antibodies with fluorescent dyes. Fluorescently labeled antibodies not only reveal the spatial distribution and dynamic changes of proteins inside and outside cells but are also widely applied in flow cytometry, fluorescence microscopy imaging, Western blotting, and high-throughput screening platforms. With the advancement of multicolor labeling techniques and high-performance fluorescent dyes, researchers can analyze multiple target molecules simultaneously within a single experiment, greatly enhancing experimental efficiency and data reliability.
Understanding Fluorescent Labeling of Antibodies
What is Antibody Fluorescent Labeling?
Antibody fluorescent labeling is a biochemical technique in which a specific antibody is covalently linked to a fluorescent dye (fluorophore) via chemical reactions, enabling both antigen recognition and fluorescence signal detection. This process preserves the antibody's high specificity for its target antigen while conferring optical properties that can be excited and detected, allowing precise localization and quantitative analysis within complex biological systems. With the development of high-throughput analysis and automated imaging, fluorescent antibody applications have expanded from single-target detection to multiplex labeling and quantitative imaging. In these applications, fluorophores with distinct spectral properties can differentiate multiple signal channels, enabling parallel analysis of complex biological systems and significantly improving experimental efficiency and data quality.
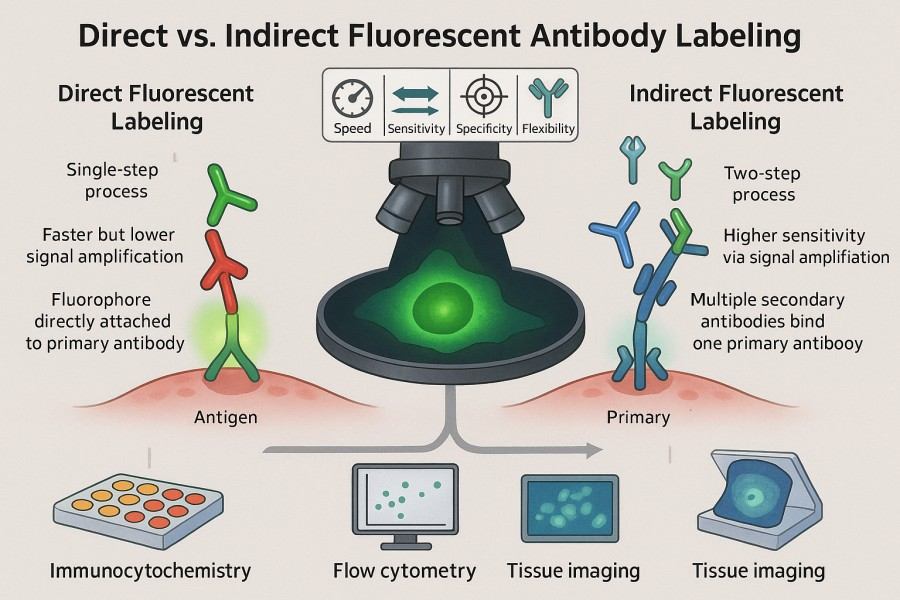 Fig. 1. Direct vs. indirect fluorescent antibody labeling (BOC Sciences Authorized).
Fig. 1. Direct vs. indirect fluorescent antibody labeling (BOC Sciences Authorized).
How Does Fluorescent Labeling Work?
The chemistry of antibody fluorescent labeling is based on reactive chemical groups present on the antibody molecule, which can be selectively covalently coupled to reactive functional groups on the fluorophore. Common reactive sites include amino groups (–NH₂) on lysine residues and thiol groups (–SH) on cysteine residues, while fluorescent dyes often carry reactive groups such as N-hydroxysuccinimide (NHS) esters, isothiocyanates, or maleimides.
During the reaction, the fluorescent dye forms a stable covalent bond with the antibody, producing a fluorescent antibody conjugate. After labeling, purification steps are required to remove free dye and by-products, ensuring signal purity and experimental reproducibility. Optimized fluorescent antibodies absorb energy at specific wavelengths and emit fluorescence within another wavelength range. When the antibody binds its target antigen, the fluorescent signal is used for detection, quantification, or imaging, enabling direct visualization of the target molecule. The key in this process is balancing fluorescence intensity with antibody activity. Over-labeling can alter the antibody structure, affecting binding affinity, while under-labeling may result in weak signals. Therefore, controlling the dye-to-protein ratio (F/P ratio), reaction time, and conditions is critical for producing high-performance fluorescent antibodies.
Key Components in Fluorescent Labeling Reactions
Fluorescent antibody labeling reactions typically involve three key components, each significantly affecting the final labeling quality and detection performance:
- Antibody: High-purity antibodies free of carrier proteins (e.g., BSA) are preferred to avoid interference with the conjugation reaction or non-specific binding. Monoclonal antibodies generally offer higher consistency and reproducibility, while polyclonal antibodies provide stronger signals when recognizing multiple antigen epitopes.
- Fluorescent Dye (Fluorophore): The choice of fluorophore directly determines the spectral properties, signal intensity, and photostability of the labeled product. Common dye types include FITC, Rhodamine, Alexa Fluor, Cyanine, and BODIPY, each offering specific advantages in excitation wavelength, quantum yield, and resistance to photobleaching.
- Buffer and Reaction Conditions: The reaction system should have an appropriate pH (typically 7.5–8.5) and ionic strength to ensure smooth chemical reactions. Excessive temperature may denature the antibody, while too low a temperature can slow the reaction rate. Common buffers include PBS, carbonate buffer, or HEPES buffer.
Precise control of these three factors enables researchers to obtain high-purity fluorescent antibodies with strong signals while maintaining biological activity, providing reliable tools for subsequent immunodetection and imaging experiments.
Principles and Methods of Antibody Labeling with Fluorescent Dyes
Fluorescent antibody labeling relies not only on fundamental chemical conjugation principles but also on selecting suitable dyes and labeling strategies according to experimental needs. A scientifically optimized labeling method ensures sufficient fluorescence intensity, preserves antibody activity, and allows signal differentiation in multiplex detection, providing a reliable foundation for downstream applications.
Reactive Groups and Conjugation Chemistry
The core of antibody labeling is forming stable covalent bonds between reactive groups on the antibody and functional groups on the fluorescent dye. Common conjugation strategies include:
- NHS Ester Conjugation: NHS esters react with free amino groups on lysine residues to form stable amide bonds. This method is simple, efficient, and widely used, suitable for most antibody types.
- Maleimide Conjugation: Maleimide groups specifically react with thiol groups on cysteine residues to form stable thioether bonds. This strategy is ideal for labeling that requires precise control over dye attachment, minimizing impact on antibody activity.
- Isothiocyanate Conjugation (e.g., FITC): Isothiocyanates react with amino groups to form stable thiourea bonds. This classic fluorescent labeling method is gentle, cost-effective, and suitable for basic research and educational experiments.
- Click Chemistry: Based on azide-alkyne or other bioorthogonal reactions, click chemistry offers high specificity, high yield, and mild conditions. It is particularly useful for multiplex labeling, precise quantification, and conjugation with proteins, nanomaterials, or drug molecules.
Careful selection of conjugation chemistry not only improves labeling efficiency but also reduces the risk of antibody structural damage and non-specific binding, providing stable and reliable experimental results.
Methods of Fluorescent Labeling Antibody (Direct vs. Indirect Fluorescent Antibody Labeling)
Fluorescent antibody labeling methods are primarily divided into direct labeling and indirect labeling. Each approach has specific characteristics regarding workflow, signal intensity, and application scenarios.
Table 1. Common methods for fluorescent antibody labeling.
| Feature | Direct Labeling | Indirect Labeling |
|---|---|---|
| Principle | Fluorophore is directly conjugated to the primary antibody. | Unlabeled primary antibody binds antigen, fluorescent secondary antibody binds primary antibody. |
| Experimental Steps | Single-step conjugation and direct application. | Multi-step: primary antibody binding → secondary antibody binding → signal detection. |
| Signal Intensity | Limited signal, fewer dyes per antibody. | Enhanced signal, multiple dyes per secondary antibody. |
| Background Signal | Lower, as secondary antibody is omitted. | Potentially higher, requires optimized blocking and washing. |
| Operation Time | Short, rapid. | Longer, more steps. |
| Suitable Applications | Rapid detection, high-abundance proteins. | Low-abundance proteins, multiplex detection, or signal amplification. |
| Cost | Higher, each primary antibody needs separate labeling. | Lower, universal secondary antibody can be used with multiple primary antibodies. |
| Multiplexing Difficulty | Higher, requires differently labeled primary antibodies. | Easier, but requires careful management of cross-reactivity. |
Factors Affecting Labeling Efficiency and Signal Stability
The efficiency and signal stability of fluorescent antibody labeling are influenced by multiple factors:
- Antibody Concentration and Purity: Low antibody concentrations can lead to uneven labeling, and high levels of impurities or carrier proteins may interfere with the conjugation reaction.
- Dye Ratio (F/P Ratio): Excess dye may cause self-quenching, while insufficient dye results in weak signals; generally, an F/P ratio between 3–8:1 is recommended.
- Buffer Composition and pH: The buffer system should avoid free amino or thiol groups that interfere with the reaction while maintaining suitable pH to ensure reactivity.
- Temperature and Reaction Time: Excessive temperature may denature the antibody, whereas insufficient reaction time reduces conjugation efficiency.
- Light Exposure and Storage Conditions: Fluorescent dyes are prone to photobleaching; therefore, reactions should be conducted in the dark, and labeled antibodies stored at low temperatures to prolong stability.
Optimizing these factors allows the production of efficient, stable fluorescently labeled antibodies while maintaining antibody activity, providing a reliable foundation for subsequent experiments.
Common Fluorescent Dyes Used in Antibody Labeling
Commonly used fluorescent dyes for antibody labeling each have unique characteristics. When selecting a dye, researchers must consider the experimental system, excitation source, detection filters, signal intensity, and multiplexing requirements. A proper choice of dye not only affects fluorescence intensity but also directly impacts experimental reproducibility and data reliability.
FITC (Fluorescein Isothiocyanate)
FITC is a classic green fluorescent dye widely used in basic flow cytometry and immunoassays due to its ease of use and low cost. FITC covalently attaches to antibody lysine residues via its isothiocyanate group, forming stable fluorescent antibodies. However, it has relatively poor photostability and can photobleach under prolonged light exposure. FITC is suitable for rapid detection and single-target analysis but may require consideration of signal decay in multiplex or high-intensity imaging experiments.
Rhodamine
Rhodamine dyes have longer emission wavelengths and good photostability, providing clear and reliable fluorescence signals in complex biological systems. Their chemical structure performs exceptionally well in confocal microscopy and multicolor imaging, making them ideal for experiments detecting multiple targets simultaneously. Rhodamine-labeled antibodies are bright and photostable, outperforming FITC in long-term imaging and high-resolution experiments, ensuring signal stability and reproducibility.
Alexa Fluor Series
Alexa Fluor dyes are known for high brightness, broad spectral coverage, and excellent photostability. Their excitation wavelengths span from ultraviolet to near-infrared, supporting multiplexing and high-sensitivity detection. Antibodies labeled with Alexa Fluor exhibit stable signals over long-term imaging studies, making them ideal for high-end fluorescence imaging, flow cytometry, and multichannel analysis. This series is compatible with diverse biological systems and experimental conditions, allowing high-quality visualization of multiple targets.
Cyanine Dyes (Cy3, Cy5, Cy7)
Cyanine dyes such as Cy3, Cy5, and Cy7 offer broad spectral ranges and high quantum yields, suitable for multichannel flow cytometry and molecular imaging. Covalent conjugation to antibodies enables simultaneous detection of multiple targets, supporting complex sample analysis. Cyanine dyes are photostable, suitable for long-term observation and high-sensitivity experiments, and reduce signal overlap in multiplex labeling, ensuring accurate signal separation. They are commonly used in high-throughput screening and multicolor imaging studies.
BODIPY Dyes
BODIPY dyes are valued for high quantum yield, sharp emission spectra, and excellent photostability. BODIPY-labeled antibodies demonstrate exceptional signal stability in high-resolution imaging and quantitative analysis, making them suitable for fine biological imaging and fluorescent probe development. Their chemical properties allow consistent brightness and stability in various biological systems, even under prolonged illumination or high-sensitivity detection, making them ideal for advanced imaging and quantitative studies.
Table 2. Common fluorescent fluorophores.
| Catalog | Name | CAS | Fluorescence Color | Ex (nm) | Em (nm) | Main Applications | Inquiry |
|---|---|---|---|---|---|---|---|
| F01-0161 | BODIPY 558/568 C12 | 158757-84-7 | Orange-Red | 558 | 568 | Labeling intracellular neutral lipids; studying lipid droplets in live cells | Bulk Inquiry |
| F01-0045 | BODIPY 505/515 | 21658-70-8 | Green | 505 | 515 | Visualizing lipid droplets in microalgae; fluorescence microscopy applications | Bulk Inquiry |
| F01-0044 | BODIPY-Cholesterol | 878557-19-8 | Green | 505 | 515 | Cholesterol trafficking studies; lipid membrane labeling | Bulk Inquiry |
| R02-0024 | Cyanine7 alkyne | 1998119-13-3 | Near-Infrared | 743 | 767 | In vivo imaging; click chemistry conjugation | Bulk Inquiry |
| F02-0006 | Cyanine3.5 carboxylic acid | 1802928-88-6 | Green | 520 | 540 | Nucleic acid and protein labeling; qPCR; RNA/DNA isolation | Bulk Inquiry |
| F03-0009 | Sulfo-Cyanine5.5 amine | 2183440-45-9 | Far-Red | 675 | 695 | In vivo imaging; antibody labeling; flow cytometry | Bulk Inquiry |
| F02-0012 | Cyanine5.5 carboxylic acid | 1144107-80-1 | Far-Red | 675 | 695 | Control/reference sample; instrument calibration | Bulk Inquiry |
| F02-0013 | Cyanine5.5 maleimide | 1594414-90-0 | Far-Red | 675 | 695 | Thiol-reactive conjugation; protein labeling | Bulk Inquiry |
| R01-0042 | AF594 activated ester, 5-isomer | 1638544-48-5 | Red | 590 | 617 | Antibody labeling; fluorescence microscopy; flow cytometry | Bulk Inquiry |
| R01-0471 | AF647 NHS ester | 407627-60-5 | Far-Red | 650 | 665 | Antibody labeling; in vivo imaging; multiplex assays | Bulk Inquiry |
| R01-0039 | AF430 NHS ester | 467233-94-9 | Blue | 430 | 440 | Antibody labeling; flow cytometry; fluorescence microscopy | Bulk Inquiry |
| R01-0451 | AF 488 TFP ester | 2133404-55-2 | Green | 495 | 519 | Antibody labeling; flow cytometry; microscopy | Bulk Inquiry |
| F05-0031 | 6-Carboxy-X-rhodamine | 194785-18-7 | Orange-Red | 580 | 605 | Protein labeling; fluorescence microscopy; flow cytometry | Bulk Inquiry |
| A16-0014 | Sulforhodamine 101 | 60311-02-6 | Red | 583 | 604 | Cell viability assays; fluorescence microscopy; flow cytometry | Bulk Inquiry |
| A16-0093 | Rhodamine 6G | 989-38-8 | Orange-Red | 530 | 552 | Fluorescence microscopy; flow cytometry; laser dye | Bulk Inquiry |
| A01-0005 | Rhodamine B | 81-88-9 | Orange-Red | 540 | 625 | Fluorescence microscopy; dye laser; protein labeling | Bulk Inquiry |
| A17-0016 | Rhodamine 6G Perchlorate | 13161-28-9 | Orange-Red | 530 | 552 | Fluorescence microscopy; flow cytometry; laser dye | Bulk Inquiry |
| A18-0008 | Rhodamine 110 chloride | 13558-31-1 | Green | 502 | 518 | Fluorescence microscopy; flow cytometry; ion channel studies | Bulk Inquiry |
| F04-0012 | FITC isomer I | 3326-32-7 | Green | 495 | 519 | Antibody labeling; flow cytometry; fluorescence microscopy | Bulk Inquiry |
| F04-0055 | Dexamethasone Fluorescein | 216854-76-1 | Green | 495 | 519 | Steroid receptor studies; fluorescence microscopy | Bulk Inquiry |
| R10-0005 | 6-Fluorescein phosphoramidite | 204697-37-0 | Green | 494 | 521 | Nucleic acid labeling; oligonucleotide synthesis | Bulk Inquiry |
| A16-0033 | 6-Carboxyfluorescein | 3301-79-9 | Green | 494 | 521 | Nucleic acid labeling; fluorescence microscopy; flow cytometry | Bulk Inquiry |
| A15-0005 | 5(6)-Carboxyfluorescein | 72088-94-9 | Green | 494 | 521 | Nucleic acid labeling; fluorescence microscopy; flow cytometry | Bulk Inquiry |
| F04-0033 | 5-Aminofluorescein | 3326-34-9 | Green | 494 | 521 | Nucleic acid labeling; fluorescence microscopy; flow cytometry | Bulk Inquiry |
Looking for Antibody Labeling Dyes?
We provide flexible conjugation options with various fluorophores, including water-soluble and photostable dyes, to meet your experimental requirements.
Workflow for Antibody Fluorescent Labeling
While the experimental workflow for antibody fluorescent labeling is based on standardized chemical conjugation principles, each step must be carefully optimized to ensure labeling efficiency, antibody activity, and signal stability. A well-designed workflow increases experimental success and ensures the reliability and reproducibility of labeled antibodies in downstream applications.
Sample Preparation and Buffer Optimization
- Antibody Pretreatment: Use highly purified antibodies and remove components that may interfere with conjugation, such as BSA, collagen, amine-containing buffers (e.g., Tris, Glycine), thiol reducing agents (e.g., DTT, β-ME), and other contaminants. Typically, antibodies are exchanged into a conjugation-compatible buffer (e.g., PBS pH 7.4 without amines, NaN₃, Glycine, or EDTA/minimal EDTA and low metal ion content) via dialysis, desalting columns, or ultrafiltration.
- Antibody Concentration Determination: Accurately measure antibody concentration (e.g., using A₂₄₀/A₂₈₀ or UV absorbance methods). A concentration ≥ 1 mg/mL is generally recommended to ensure sufficient reaction capacity.
- Buffer System Selection: Commonly used pH ranges are 7.2–8.5, depending on the dye and conjugation chemistry. Avoid buffers containing NH₂ or SH groups. Small amounts of NaHCO₃ or Na₂HPO₄ can be added to stabilize the reaction environment.
- Dye Pretreatment: Depending on the dye type, dissolve it in a small volume of DMSO or DMF and quickly add it to the antibody mixture. Avoid long-term storage of dyes in aqueous solutions to prevent hydrolysis.
- Reaction System Adjustment: Set appropriate D/P ratios, reaction volume, temperature (usually room temperature or 4 °C), reaction time (30 min–2 h), and gentle mixing.
Dye–Antibody Conjugation Steps
- Place the pretreated antibody in the conjugation buffer.
- Add the activated dye (e.g., NHS ester or maleimide) and gently mix within the reaction system.
- Allow the reaction to proceed while controlling temperature and time; avoid strong light to minimize dye photobleaching.
- Stop the reaction by adding an amine-containing buffer (e.g., Tris) to quench remaining active sites, or add glycine for blocking.
- Minimize dye self-quenching or antibody conformational damage by proceeding to purification promptly after the reaction.
Purification and Quality Validation Methods
- Removal of Unconjugated Dye: Eliminate free dye using centrifugal ultrafiltration devices (e.g., 10–30 kDa cutoff), gel filtration columns (e.g., Sephadex G-25), or dialysis.
- D/P Ratio Measurement: Determine dye-to-protein ratio by measuring absorbance at 280 nm (antibody) and the dye's characteristic wavelength. Ensure the D/P ratio is within an appropriate range (typically 2–8, depending on dye and application).
- Antibody Activity Assay: Verify that the conjugated antibody still recognizes its target antigen using ELISA, Western blot, or flow cytometry.
- Fluorescence Spectrum Analysis: Confirm that the excitation/emission spectra of the conjugated antibody meet expectations and that fluorescence intensity meets downstream detection requirements. Measurements can be performed using a fluorometer or fluorescence imaging equipment.
- Stability Testing: Assess the labeled antibody's stability by storing it short-term (e.g., 1 week at 4 °C in the dark) and then re-measuring fluorescence intensity and antibody activity.
Advantages of Fluorescent Conjugated Antibodies
Fluorescently labeled antibodies combine high specificity with sensitive signal detection, enabling multi-layered, multidimensional biological analysis. Compared to traditional enzymatic or radioactive labeling methods, they offer significant technical and practical advantages:
- High Sensitivity and Rapid Detection: Fluorescent antibodies generate detectable signals even at low target concentrations, greatly improving experimental sensitivity. Fluorescence can be directly detected by lasers or microscopes without additional substrate reactions, allowing faster experiments suited for high-throughput screening and real-time analysis.
- Multiplexing and Complex System Analysis: By selecting dyes with different wavelengths, multiple target proteins can be labeled simultaneously for multichannel detection. Multiplexing is particularly valuable in complex cell systems, tissue sections, or multi-protein complex studies, enhancing efficiency and data richness.
- Non-Destructive Detection and Dynamic Tracking: Fluorescent antibodies enable live-cell or tissue imaging, observing target molecules in their native state. Unlike chemical stains or radioactive labels, they do not damage samples and are suitable for protein dynamics, signaling pathway tracking, and drug target validation.
- Quantitative Capability and High-Precision Analysis: Fluorescence intensity correlates linearly with protein abundance, enabling precise quantitative analysis. This is crucial for flow cytometry, imaging quantification, and high-throughput screening, supporting scientific decision-making and drug development.
- Broad Application and Experimental Compatibility: Fluorescent antibodies are compatible with flow cytometers, fluorescence microscopes, confocal microscopes, and high-throughput automated systems. Their versatility allows the same batch to be used across different experiments, improving research and diagnostic efficiency and cost-effectiveness.
Common Challenges in Antibody Fluorescent Labeling
Despite their advantages, fluorescently labeled antibodies face several challenges during experimental use. Scientists must understand potential issues and adopt strategies to optimize performance, signal stability, and reproducibility.
- Maintaining Antibody Activity and Specificity: Antibody binding activity is critical for successful labeling. Over-labeling or inappropriate chemical conjugation may alter the antibody's conformation, affecting antigen recognition. Monoclonal antibodies are particularly sensitive to structural changes. Dye-to-antibody molar ratios should be controlled, extreme pH or high temperatures avoided, and mild buffers used to preserve high specificity after labeling.
- Over-Labeling and Signal Quenching Issues: Excessive dye can damage antibody structure and cause self-quenching, reducing fluorescence intensity. This is particularly notable with high-quantum yield dyes like FITC or Rhodamine. Optimizing the F/P ratio, typically 3–8:1, is key to preventing these issues.
- Managing Dye-to-Protein Ratios (F/P Ratio): The F/P ratio directly affects fluorescence intensity and antibody activity. Too low results in weak signals, too high can damage the antibody or cause self-quenching. Accurate calculation and measurement of the F/P ratio using UV-Vis or fluorescence assays is essential for balancing signal intensity and antibody activity.
- Fluorophore Compatibility and Cross-Talk in Multiplex Labeling: In multiplex experiments, dyes may have spectral overlap, causing cross-talk and data confusion. Selecting dyes with distinct spectra and strong photostability, along with proper filter and excitation design, minimizes interference. Avoiding chemical reactions or competition between dyes is also crucial for clear multichannel signals.
- Reproducibility and Batch-to-Batch Variability: Experimental reproducibility is affected by antibody purity, dye batch, conjugation conditions, and operator variability. Standardizing workflows, controlling buffers and reaction conditions, and using the same batch of antibodies or dyes for critical experiments improve reproducibility. Maintaining experimental records and quality control standards ensures long-term batch consistency.
- Storage Stability and Shelf Life of Labeled Antibodies: Storage conditions critically affect signal stability. Fluorescent dyes are sensitive to photobleaching, oxidation, and temperature fluctuations, which can weaken signals or degrade antibodies. It is generally recommended to store in the dark at 4°C or –20°C with stabilizers (e.g., glycerol, BSA), avoiding repeated freeze-thaw cycles or prolonged room temperature exposure to preserve antibody activity and fluorescence.
How to Select the Right Fluorophore for Your Antibody Labeling Project?
Selecting the appropriate fluorophore is critical to the success and data quality of antibody-based fluorescence experiments. Different dyes vary significantly in spectral properties, photostability, quantum yield, and compatibility with multiplex labeling. Therefore, careful screening and optimization during project design are essential.
Spectral Properties and Compatibility Considerations
When choosing a fluorescent dye, the first consideration is the match between excitation and emission wavelengths. The dye should be compatible with the light source, filters, and detectors used in the experiment to maximize signal while minimizing background. For example, FITC is suitable for 488 nm excitation, whereas Alexa Fluor 647 is suitable for near-infrared excitation. In multi-channel experiments, dye combinations with sufficient spectral separation should be selected to avoid spectral overlap and misinterpretation, ensuring accurate multiplex detection.
Single vs. Multiplex Labeling Strategies
In experimental design, single labeling is suitable for applications with a clear target and simple signal detection, while multiplex labeling is used to detect multiple target proteins simultaneously. Multiplex labeling requires consideration of dye spectral separation, matched quantum yields, and compatibility with excitation sources. Proper design of dye combinations and detection channels maximizes data output while maintaining signal clarity and antibody activity, suitable for flow cytometry, multicolor microscopy, and high-throughput screening.
Stability and Photobleaching Resistance
Fluorescent dyes are prone to photobleaching under excitation light, which can lead to signal decay and data distortion. Selecting dyes with high photostability and quantum yield, such as Alexa Fluor or BODIPY, can significantly extend imaging time and experiment reproducibility. For long-term imaging or live-cell tracking, photostable dyes maintain consistent signals, reduce experimental errors, and improve data reliability.
Additional Considerations
Beyond spectral properties and stability, the impact of the dye on antibody structure, compatibility with conjugation chemistry, and experimental cost should be considered. Some dyes may affect antibody activity during conjugation, so mild conjugation conditions are preferred. For high-throughput projects, reproducibility and batch-to-batch consistency of the dye are also critical. By comprehensively evaluating these factors, researchers can select the optimal fluorophore for their specific experiments, achieving efficient and reliable antibody labeling and detection.
Applications of Fluorescently Labeled Antibodies
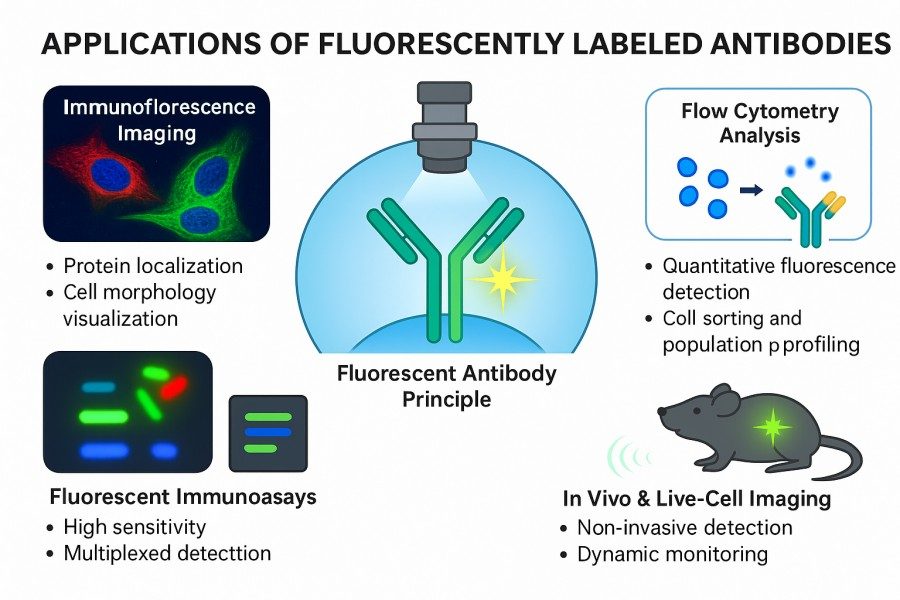 Fig. 2. Applications of fluorescently labeled antibodies (BOC Sciences Authorized).
Fig. 2. Applications of fluorescently labeled antibodies (BOC Sciences Authorized).
Fluorescent-Labeled Antibodies for Flow Cytometry
Fluorescently labeled antibodies allow rapid detection of thousands to tens of thousands of individual cells in flow cytometry, enabling precise analysis of surface and intracellular protein expression. Multichannel fluorescence labeling permits simultaneous detection of multiple antigens, facilitating immunophenotyping of complex cell populations, such as distinguishing lymphocyte subsets or assessing immune activation status. This technique is widely used in basic research, immune monitoring, and drug development studies.
Fluorescent-Labeled Antibodies for Live Cell Imaging
In tissue sections or live cell imaging, fluorescently labeled antibodies can visualize disease-associated cell populations or protein expression. By combining multicolor fluorescence labeling with high-resolution imaging platforms, quantitative analyses of tumor microenvironments, inflammatory responses, and drug targeting efficacy can be performed, providing intuitive and quantifiable data for mechanistic studies and drug development.
Fluorescent-Labeled Antibodies for Immunofluorescence Imaging
Fluorescently labeled antibodies enable direct visualization of protein distribution and cellular structures under a microscope. Coupled with confocal or live-cell imaging, researchers can generate high-resolution 3D images and track protein dynamics within organelles or tissues. This is valuable for studying organelle function, signal pathway regulation, and protein relocalization under pathological conditions.
Fluorescent-Labeled Antibodies for High-Throughput Screening
In drug development or gene function studies, fluorescent antibodies can be applied to automated high-throughput platforms for rapid, parallel analysis of thousands to tens of thousands of samples. Their sensitivity, quantifiability, and multichannel detection capabilities make them core tools for drug target validation, candidate drug screening, and functional gene analysis, greatly enhancing screening efficiency and experimental reliability.
Fluorescent-Labeled Antibodies for Western Blotting
Fluorescent antibodies provide higher sensitivity and dynamic range than enzyme-labeled secondary antibodies in Western blotting. Multiplex fluorescence labeling allows simultaneous detection of multiple proteins on a single membrane, avoiding repeated experiments. The ability to image directly without substrate reactions improves detection efficiency and reduces background interference, making it suitable for accurate protein quantification and signal comparison.
Fluorescent-Labeled Antibodies for Protein Localization Analysis
Fluorescent antibodies reveal the spatial distribution of proteins within cells or tissues and allow tracking of their migration or aggregation under specific conditions. Using dual or multiple labeling strategies, protein complexes or signaling pathway interactions can be analyzed, aiding researchers in understanding molecular mechanisms, signal transduction networks, and cellular function regulation.
Fluorescent-Labeled Antibodies for Molecular Diagnostics
Fluorescent antibodies are widely used in clinical diagnostics. Fluorescent immunoassays can sensitively detect pathogens and tumor markers in blood, tissue, or bodily fluids. Their rapid, accurate, and quantifiable nature is valuable for early disease diagnosis, therapy monitoring, and personalized treatment planning, significantly improving clinical detection efficiency and reliability.
Fluorescent-Labeled Antibodies for Cell Sorting
Fluorescent antibodies are used in fluorescence-activated cell sorting (FACS) to identify and isolate specific cell subpopulations. Labeled target cells can be sorted at high purity for stem cell research, immunotherapy development, or downstream functional assays. By combining multicolor labeling with precise threshold settings, high-precision, low-contamination target cell collection is achievable, providing reliable samples for subsequent experiments.
How BOC Sciences Supports Antibody Fluorescent Labeling?
BOC Sciences is committed to providing professional, high-performance fluorescent antibody labeling services for research institutions, drug development companies, and diagnostic laboratories. Leveraging years of experience in fluorescent conjugation, we offer fully customized services covering dye selection, chemical modification, conjugation design, purification, and quality validation, ensuring each batch of labeled antibodies delivers stable signals, high specificity, and reproducibility.
FITC and Rhodamine-Antibody Labeling Services
- Custom Conjugation Chemistry: Multiple conjugation chemistries are available based on antibody type and function, including FITC isothiocyanate reactions, Rhodamine active ester coupling, and lysine/cysteine-directed labeling under mild conditions.
- Dye Modification Capabilities: FITC and Rhodamine dyes can be functionalized, such as carboxyl or amino derivatization, to enhance conjugation efficiency or solubility.
- Precise F/P Ratio Control: Optimized reaction conditions and molar ratios balance fluorescence intensity and antibody activity.
- Efficient Purification and Quality Control: Gel filtration, chromatography, and HPLC remove free dyes, ensuring clear antibody signals, low background, and batch-to-batch consistency.
Alexa Fluor-Antibody Labeling Services
- Multiplex-Compatible Conjugation Design: Dye combinations optimized for multicolor experiments to avoid spectral overlap and cross-talk, suitable for flow cytometry and confocal microscopy.
- Photostability Optimization: Dye selection and conjugation positioning enhance resistance to photobleaching for long-term imaging and dynamic observation.
- Directed Conjugation and Dye Modification: Alexa Fluor dyes can be chemically modified (e.g., PEGylation, carboxylation) to improve solubility and conjugation stability.
- Strict Quality Validation: Each batch undergoes F/P ratio measurement, activity testing, and fluorescence intensity assessment to ensure reliable experimental data.
Cyanine-Antibody Labeling Services
- Efficient Multiplex Conjugation Strategies: Specialized protocols for Cy3, Cy5, and Cy7 enable clear, non-interfering signals in multichannel experiments.
- Dye Modification Capabilities: PEGylation or amino derivatization of Cy dyes improves solubility, reduces aggregation, and enhances conjugation efficiency.
- Precise Purification and F/P Control: Multi-step purification removes unbound dyes, while molar ratios are precisely controlled to optimize signal and antibody activity.
- Batch Consistency and Stability: Standardized protocols and QC ensure consistent antibody performance across batches.
BODIPY-Antibody Labeling Services
- High-Resolution Conjugation: Optimized conjugation strategies for BODIPY's small size and photostability ensure precise dye placement and uniform signal.
- Dye Chemical Modification: Functionalization, such as carboxylation, amination, or PEGylation, enhances conjugation efficiency and solubility for complex imaging experiments.
- Customized F/P Ratio and Conjugation Positioning: Optimal F/P ratios and attachment sites are designed according to antibody structure to balance fluorescence intensity and activity.
- Purification and Rigorous QC: Multi-layer purification including gel filtration, HPLC, and SDS-PAGE ensures low-background, highly specific, and reproducible labeled antibodies.
Do You Need A Consultation?
BOC Sciences integrates cutting-edge fluorescence technologies to accelerate your research, driving next-generation solutions for drug discovery and diagnostics.
Transform Your Studies with Cutting-Edge Fluorescent Products
| Cat. No. | Product Name | CAS No. | Inquiry |
|---|---|---|---|
| R01-0009 | BDY FL, SE | 146616-66-2 | Bulk Inquiry |
| A16-0170 | Rhodamine-123 | 62669-70-9 | Bulk Inquiry |
| A19-0040 | Hoechst 33342 | 23491-52-3 | Bulk Inquiry |
| A19-0103 | SYBR Green I | 163795-75-3 | Bulk Inquiry |
| A14-0036 | Rhodamine B hydrazide | 74317-53-6 | Bulk Inquiry |
| F06-0011 | Coumarin 153 | 53518-18-6 | Bulk Inquiry |
| A17-0009 | LDS 867 | 106025-71-2 | Bulk Inquiry |
| F02-0026 | Cy5-NHS ester | 146368-14-1 | Bulk Inquiry |
| F01-0251 | BODIPY 576/589 | 150173-78-7 | Bulk Inquiry |
| F02-0007 | Cyanine5 amine | 1807529-70-9 | Bulk Inquiry |
High-Performance Fluorescent Tools for Your Research
- TAMRA Dyes Red-emitting dyes for antibody and protein labeling.
- ATTO Dyes High-performance dyes for labeling and imaging experiments.
- Coumarin Blue-emitting dyes for chemical sensing and fluorescence studies.
- Fluorescent Protein Used for live-cell imaging and real-time biosensing.
More About Antibody labeling
Online Inquiry

