Direct vs. Indirect Fluorescent Antibody Labeling: Which Is Right for Your Research?
Fluorescent labeled antibodies have become indispensable tools in modern life science research, offering high sensitivity and visualization capabilities for protein detection, cell imaging, and multiplexed analysis. Whether in basic research or clinical diagnostics, scientists rely on these labeled antibodies for accurate identification and quantification of target molecules. In experimental design, choosing between direct fluorescent antibody labeling and indirect fluorescent antibody labeling is a critical decision, as each approach presents distinct advantages and limitations in terms of sensitivity, specificity, operational complexity, and signal stability. By thoroughly understanding the principles and applications of both methods, researchers can optimize their strategies to achieve more efficient and reliable protein detection and visualization outcomes.
What is Fluorescent Antibody Labeling?
Fluorescent antibody labeling refers to the technique of covalently attaching fluorescent dyes to antibodies, taking advantage of the antibody's high specificity toward its target antigen to visualize and quantify specific biomolecules within a sample. In a typical immunoassay, the antibody (a Y-shaped glycoprotein) recognizes and binds to its target antigen, and the attached fluorescent probe is then excited to emit detectable light signals. Each fluorescent dye absorbs photons (excitation light) and emits light at a longer wavelength (emission light) through a repeated excitation–emission process that can occur thousands of times, generating measurable signal intensity. Because each antibody molecule can typically bind multiple fluorescent dyes (usually around 2–7), the fluorescence signal is amplified, greatly enhancing detection sensitivity. Overall, antibody–fluorophore conjugation enables researchers to directly observe and quantify target proteins, carbohydrates, and other biomarkers in samples using instruments such as confocal microscopes, flow cytometers, or fluorescence plate readers.
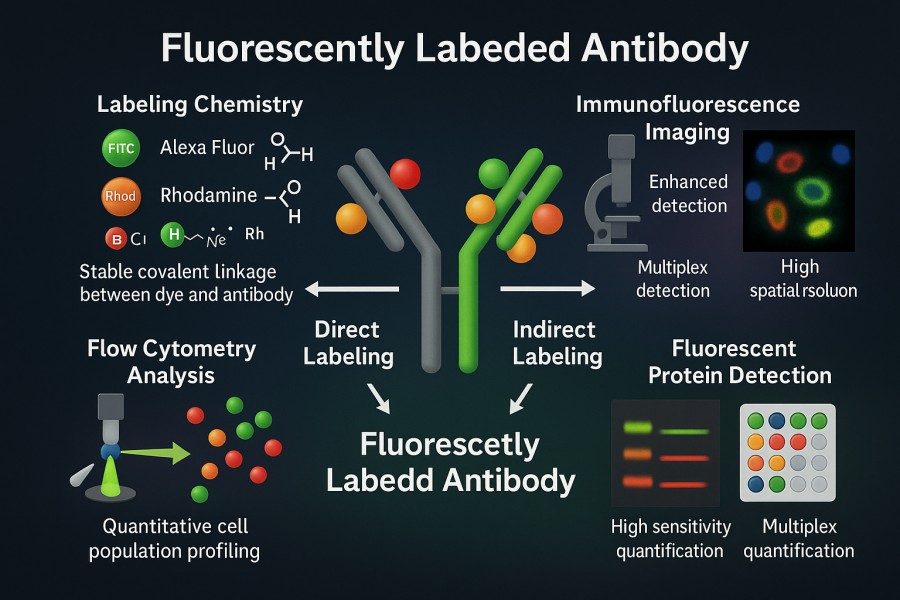 Fig. 1. Fluorescent labeled antibodies (BOC Sciences Authorized).
Fig. 1. Fluorescent labeled antibodies (BOC Sciences Authorized).
The Principles of Fluorescence Detection in Immunoassays
Fluorescence detection is based on the spectral properties of molecular absorption and emission. Fluorophores absorb photons of specific wavelengths, bringing the molecule to an excited state, and subsequently emit photons as they return to the ground state. Because some energy is lost during this process, the emitted light has a longer wavelength than the excitation light—a phenomenon known as the Stokes shift.
In immunoassays, each fluorophore undergoes this excitation–emission cycle repeatedly upon illumination, resulting in cumulative fluorescence signals. Moreover, since a single antibody can carry multiple fluorophores, the signal from one molecule can be significantly amplified. This optical amplification effect gives fluorescence detection much higher sensitivity than conventional chromogenic staining methods. To obtain quantitative data, instruments such as microscopes, plate readers, or flow cytometers use preset filters and detectors to measure the emission intensity of each fluorophore. It is essential to ensure that the fluorescent conjugation does not interfere with the antibody's antigen-binding sites, as this could compromise binding specificity.
How Fluorescent Conjugation Enables Protein Visualization and Quantification?
Once an antibody is conjugated with a fluorophore, the target protein's spatial distribution can be directly visualized under a fluorescence microscope in cells or tissue sections. By exciting the fluorophore and recording the emitted light, researchers can visualize target localization in specific organelles or tissue regions. Furthermore, fluorescence intensity measurements enable the quantitative determination of target abundance.
For example, flow cytometry can measure fluorescence intensity at the single-cell level to quantify protein expression, while fluorescence-linked immunosorbent assays (FLISA) can measure well fluorescence to determine antigen concentration. Because fluorescence intensity is proportional to the antibody—and consequently to antigen—quantity, generating a standard curve allows for highly sensitive and precise quantification. Moreover, by using fluorescent dyes with different emission wavelengths (multiplex labeling), researchers can simultaneously detect multiple targets, facilitating studies on co-localization and protein interaction within cells or tissues.
What is Direct Fluorescent Antibody Labeling?
Direct fluorescent antibody labeling refers to the chemical conjugation of fluorescent molecules directly to the primary antibody. In this method, the sample is incubated with a primary antibody already labeled with a fluorophore, which binds to the target antigen and emits fluorescence directly—eliminating the need for a secondary antibody. This streamlines the workflow, making the procedure faster and simpler. Direct labeling is often used in rapid detection of bacteria or viruses and offers several benefits, including operational simplicity, shorter staining time, and high specificity. The typical experimental steps include preparing a directly labeled primary antibody solution, incubating it with fixed or unfixed cells/tissue samples, washing off excess antibodies, and visualizing the fluorescence directly under a microscope or other detection instruments.
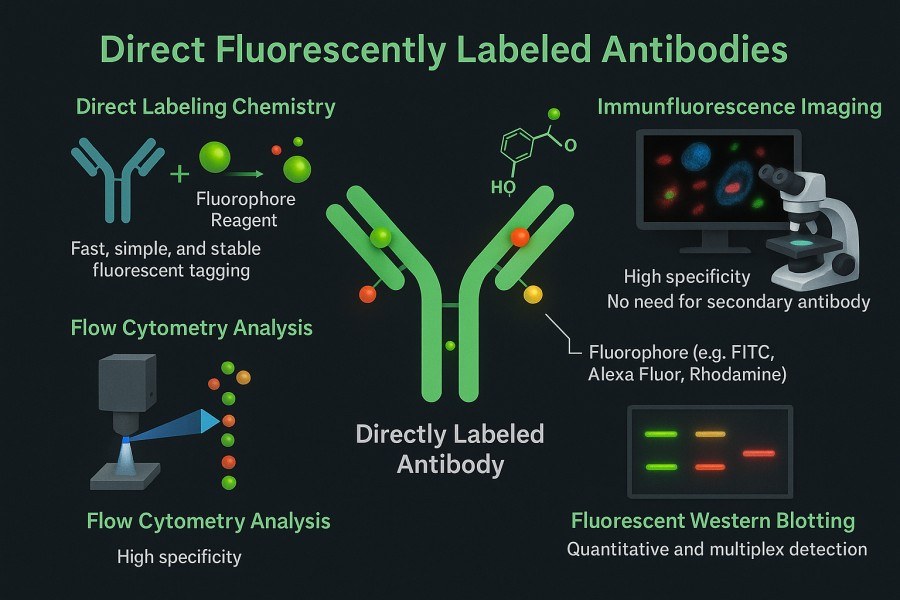 Fig. 2. Direct fluorescently labeled antibodies (BOC Sciences Authorized).
Fig. 2. Direct fluorescently labeled antibodies (BOC Sciences Authorized).
Commonly Used Fluorophores for Direct Labeling
Selecting an appropriate fluorescent dye is critical for ensuring signal quality and experimental reliability in direct antibody labeling. Different fluorophores vary in emission spectra, photostability, and chemical reactivity, allowing researchers to choose based on their detection system, instrumentation, and desired signal strength.
- FITC (Fluorescein Isothiocyanate): One of the earliest and most widely used fluorescent dyes, FITC emits green fluorescence with an excitation wavelength of ~492 nm and an emission wavelength of ~520 nm. Its isothiocyanate group reacts readily with amine residues on antibodies to form stable thiourea bonds, making it simple and highly compatible for cell imaging, immunofluorescence, and flow cytometry. However, FITC is prone to photobleaching and is best suited for short-term imaging experiments under low-light conditions.
- Alexa Fluor Series Dyes: Alexa Fluor dyes represent a new generation of high-performance fluorophores, covering the full spectral range from blue (Alexa Fluor 350) to far-red (Alexa Fluor 750). Compared with FITC, Alexa Fluor dyes exhibit higher photostability, quantum yield, and resistance to photobleaching, making them ideal for long-term live-cell imaging and multichannel detection. Their reactive groups—such as NHS esters, maleimides, and azides—enable efficient and controllable conjugation, making them the mainstream choice for protein labeling and multiplex immunoassays.
- Cy Series Dyes (e.g., Cy3, Cy5, Cy7): The Cy dye family is characterized by strong brightness, high signal intensity, and excellent spectral separation. Cy3 (orange-red) and Cy5 (far-red) are commonly used in dual or multiplex labeling experiments, allowing simultaneous detection of multiple targets. With their longer emission wavelengths, Cy dyes minimize background autofluorescence and are well suited for fluorescence resonance energy transfer (FRET) studies, protein–protein interaction assays, and signal detection in complex biological samples.
Advantages — Simplicity, Reduced Background, Fast Detection
Direct fluorescent antibody labeling offers a straightforward and time-efficient approach that minimizes experimental complexity while ensuring reliable signal detection.
- Streamlined workflow: Direct labeling requires only one antibody incubation step, eliminating the need for a secondary antibody and additional washing.
- Time efficiency: The overall staining process is faster, making it ideal for high-throughput or time-sensitive experiments.
- Avoids species cross-reactivity: When multiple primary antibodies originate from the same host species, direct labeling helps prevent species conflict in multiplex assays.
- Reduced non-specific background: Since no secondary antibody is used, background fluorescence is typically lower, minimizing unintended binding to sample components.
- Ideal for high-abundance targets: Directly labeled antibodies perform well in localization studies or when antigen expression levels are relatively high.
Limitations — Signal Intensity, Photobleaching, and Labeling Efficiency
Despite its simplicity, direct labeling also presents several drawbacks that may limit its use in experiments requiring high signal sensitivity or long-term imaging.
- Limited signal amplification: Each antibody can carry only a finite number of dye molecules, resulting in weaker fluorescence compared to indirect labeling.
- Poor detection of low-expression targets: For antigens expressed at very low levels, direct labeling may yield insufficient or undetectable signal intensity.
- Photobleaching concerns: Some classic dyes (e.g., FITC) have low photostability and may fade quickly during prolonged imaging or exposure.
- Higher cost and limited availability: Fluorescently labeled primary antibodies are often more expensive and available in fewer varieties than unlabeled ones.
- Increased experimental complexity for custom labeling: When a desired conjugate is unavailable commercially, researchers must perform in-house labeling using conjugation reagents, adding extra workload and cost.
What is Indirect Fluorescent Antibody Labeling?
Indirect fluorescent antibody labeling involves using an unlabeled primary antibody to first bind to the antigen, followed by a fluorescently labeled secondary antibody that recognizes and binds to the primary antibody for signal detection. Specifically, the secondary antibody is typically an anti-IgG raised against the host species of the primary antibody (e.g., goat anti-mouse, donkey anti-rabbit), and it is conjugated with a fluorescent dye. The experimental workflow includes: incubating the sample with the unlabeled primary antibody to bind the target, then incubating it with the fluorescently labeled secondary antibody to allow binding to the primary antibody, and finally exciting and visualizing the fluorescence under a microscope or detection instrument. Although this two-step method is more time-consuming, it provides signal amplification and greater experimental flexibility.
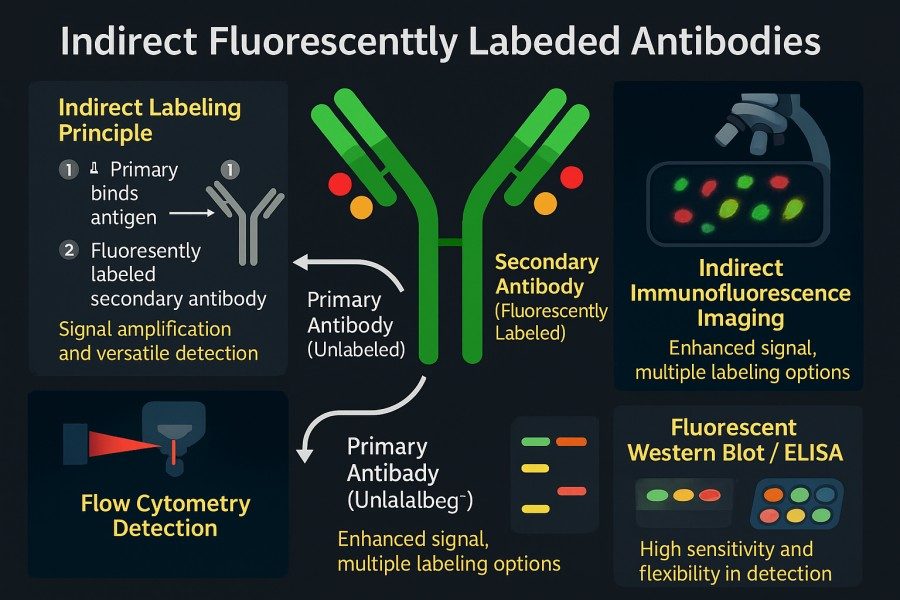 Fig. 3. Indirect fluorescently labeled antibodies (BOC Sciences Authorized).
Fig. 3. Indirect fluorescently labeled antibodies (BOC Sciences Authorized).
Common Fluorescent Secondary Antibodies and Their Roles
- Fluorescent secondary antibodies play crucial roles in indirect labeling systems by amplifying signals and ensuring specific recognition. They are typically polyclonal antibodies raised against the immunoglobulin (IgG) of the primary antibody's host species and conjugated to a specific fluorescent dye to visualize the target antigen bound by the primary antibody.
- Common types of fluorescent secondary antibodies include those targeting IgGs from various species, such as goat anti-mouse IgG, goat anti-rabbit IgG, donkey anti-goat IgG, and donkey anti-chicken IgY. These secondary antibodies are most often derived from goats or donkeys to minimize immune cross-reactivity with endogenous antibodies commonly found in mammalian samples. Choosing the right secondary antibody requires matching the host species of the primary antibody, as well as considering sample type and the possibility of nonspecific binding.
- Fluorescent secondary antibodies are frequently conjugated with dyes of different wavelengths, such as Alexa Fluor 488 (green fluorescence, Ex 495 nm/Em 519 nm), Alexa Fluor 594 (red fluorescence, Ex 590 nm/Em 617 nm), Alexa Fluor 647 (far-red fluorescence, Ex 650 nm/Em 668 nm), Cy3 (orange-red, Ex 550 nm/Em 570 nm), and Cy5 (far-red, Ex 649 nm/Em 670 nm). For example, goat anti-mouse IgG Alexa Fluor 488 refers to a goat-derived anti-mouse IgG antibody conjugated with Alexa Fluor 488 dye, which emits bright green fluorescence when excited by a laser at approximately 495 nm—allowing visualization of target proteins labeled by mouse primary antibodies.
- To improve specificity and reduce nonspecific signals, high-quality commercial secondary antibodies are often cross-adsorbed, meaning they are treated with sera from multiple non-target species during production to remove potential cross-reactivity with other immunoglobulins. This process is particularly valuable in multiplex immunolabeling experiments, where it significantly minimizes background noise and enhances detection accuracy.
- Additionally, researchers may select different antibody fragment types based on experimental needs. For example, F(ab')₂ fragment secondary antibodies, which lack the Fc region, can effectively reduce background staining caused by Fc receptor binding—making them ideal for cell surface labeling and tissue immunostaining applications.
Advantages — Signal Amplification, Versatility, Cost-Effectiveness
The advantages of indirect fluorescent antibody labeling lie primarily in its signal enhancement and experimental flexibility:
- Significant signal amplification: Each primary antibody molecule can be recognized and bound by multiple secondary antibodies, each carrying several fluorophore molecules. This dramatically increases fluorescence intensity and makes the method particularly sensitive for detecting low-abundance targets.
- High detection sensitivity: The enhanced fluorescence output allows for the detection of trace target molecules that may be undetectable by direct labeling methods, effectively lowering the detection limit.
- Excellent cost-efficiency: Fluorescently labeled secondary antibodies are generally less expensive and more versatile. A single batch of secondary antibody can be used with various primary antibodies of the same host species, reducing the need for repeated labeling preparations.
- Flexible experimental design: Researchers can couple different fluorescent dyes to the same type of secondary antibody for multichannel detection, or use secondary antibodies with different emission wavelengths to combine multiple primary antibodies in multiplex immunostaining experiments.
- Broad application compatibility: This method is suitable for a wide range of applications, including immunofluorescence, Western blotting, and flow cytometry, and can be easily integrated into existing detection workflows.
Limitations — Longer Protocols, Non-Specific Binding, Cross-Reactivity
Despite its advantages in signal enhancement, the indirect labeling method also has several limitations that should be carefully considered during experimental design:
- Longer protocols: The procedure involves sequential incubation with primary and secondary antibodies, along with multiple washing steps, which increases the overall duration and complexity of the experiment.
- Increased nonspecific background: Additional binding and washing steps can result in higher background fluorescence, particularly in tissue samples containing endogenous immunoglobulins.
- Risk of cross-reactivity: When multiple primary antibodies from the same host species are used for multiplex staining, secondary antibodies that are not carefully distinguished may cross-react, leading to signal interference.
- Potential non-target binding: Secondary antibodies may bind nonspecifically to non-target antigens or other proteins within the sample, requiring appropriate controls (such as secondary-only controls) to verify and exclude false signals.
- Signal uniformity dependent on antibody quality: The quality of secondary antibody preparation and labeling directly affects fluorescence intensity and specificity. Uneven labeling ratios or incomplete purification may cause signal variability.
Common Fluorescent Dyes for Antibody Labeling
| Catalog | Name | CAS | Fluorescence Color | Ex (nm) | Em (nm) | Main Applications | Inquiry |
|---|---|---|---|---|---|---|---|
| F04-0012 | FITC isomer I | 3326-32-7 | Green | 492 | 520 | Protein labeling, immunohistochemistry, flow cytometry | Bulk Inquiry |
| F02-0026 | Cy5-NHS ester | 146368-14-1 | Far-red | 649 | 670 | Protein/antibody labeling, multiplex imaging | Bulk Inquiry |
| A16-0004 | Phalloidin-FITC | 915026-99-2 | Green | 496/516 | 516 | Cytoskeleton staining, F-actin visualization | Bulk Inquiry |
| A16-0002 | Phalloidin-TRITC | 915013-10-4 | Red-orange | 550 | 573 | Cytoskeleton staining, F-actin visualization | Bulk Inquiry |
| F02-0030 | Cy3-NHS ester | 146368-16-3 | Orange | 550 | 570 | Protein/antibody labeling, immunofluorescence | Bulk Inquiry |
| F04-0036 | Fluorescein isothiocyanate-dextran | 60842-46-8 | Green | 495 | 525 | Intracellular tracking, vascular permeability studies | Bulk Inquiry |
| F04-0026 | Fluorescein-6-isothiocyanate | 18861-78-4 | Green | 488 | 525 | Protein labeling, immunohistochemistry, flow cytometry | Bulk Inquiry |
| F04-0032 | FAM isothiocyanate (FITC), 5- and 6-isomers | 27072-45-3 | Green | 492 | 514 | Nucleic acid probes, real-time PCR, gene expression analysis | Bulk Inquiry |
| R01-0019 | Cyanine5 NHS ester | 350686-88-3 | Far-red | 649 | 670 | Protein/antibody labeling, multiplex imaging | Bulk Inquiry |
| F03-0012 | Sulfo-Cyanine5.5 maleimide | 2183440-58-4 | Far-red | 677 | 694 | Protein/antibody labeling, multiplex imaging | Bulk Inquiry |
| R01-0039 | AF430 NHS ester | 467233-94-9 | Blue-green | 430 | 530 | Multiplex labeling, immunohistochemistry, flow cytometry | Bulk Inquiry |
| R01-0469 | AF 647 NHS ester | 1620475-28-6 | Far-red | 650 | 665 | Multiplex labeling, immunohistochemistry, flow cytometry | Bulk Inquiry |
| R01-0451 | AF 488 TFP ester | 2133404-55-2 | Green | 495 | 519 | Multiplex labeling, immunohistochemistry, flow cytometry | Bulk Inquiry |
| F02-0007 | Cyanine5 amine | 1807529-70-9 | Far-red | 649 | 670 | Protein/antibody labeling, multiplex imaging | Bulk Inquiry |
| F02-0008 | Cyanine5 azide | 1267804-34-1 | Far-red | 649 | 670 | Click chemistry labeling, protein labeling | Bulk Inquiry |
| F02-0048 | Cyanine5 carboxylic acid | 1032678-07-1 | Far-red | 649 | 670 | Protein/antibody labeling, multiplex imaging | Bulk Inquiry |
Looking for Antibody Labeling Dyes?
We provide flexible conjugation options with various fluorophores, including water-soluble and photostable dyes, to meet your experimental requirements.
Direct vs. Indirect Labeling: A Side-by-Side Comparison
Selecting the appropriate fluorescent antibody labeling strategy is critical to experimental success. Both direct and indirect labeling methods have their own advantages and limitations, and researchers must carefully consider factors such as sensitivity, specificity, experimental duration, sample type, and target characteristics to make the optimal choice.
Comparison Table: Sensitivity, Specificity, and Workflow Time
| Parameter | Direct Labeling | Indirect Labeling |
|---|---|---|
| Sensitivity | Suitable for high to moderate abundance targets (no signal amplification, relatively weaker signal). | Suitable for low-abundance targets (signal amplification provides higher sensitivity). |
| Specificity | Directly conjugated to the primary antibody, reducing cross-species reactivity. | Secondary antibody may bind to other IgGs in the sample, increasing the risk of cross-reactivity. |
| Workflow Time | Single-step incubation; primary antibody carries the fluorophore directly, fewer steps, shorter duration. | Requires sequential incubation with primary and secondary antibodies; more steps, longer overall workflow. |
In the comparison above, sensitivity is generally higher for indirect labeling due to signal amplification. Direct labeling relies only on fluorophores conjugated to a single primary antibody, resulting in a higher detection limit, making it less suitable for extremely low-abundance targets. Regarding specificity, direct labeling avoids the involvement of a secondary antibody, thereby reducing cross-binding to non-target antibodies and minimizing background. In contrast, indirect labeling introduces an additional secondary antibody, which may recognize other proteins in the sample and increase background fluorescence. In terms of workflow duration, direct labeling usually requires only a single antibody incubation, making the process simpler and faster, whereas indirect labeling requires sequential incubation and multiple washes, extending experimental time.
Choosing Based on Application Type (Cell Imaging, Western Blotting, Flow Cytometry)
- For flow cytometry, which demands high-throughput and rapid staining, direct labeling is commonly used because pre-labeled primary antibodies allow quick processing of large cell populations, and homologous primary antibodies can support multiplexing.
- For fluorescence microscopy or Western blotting targeting low-expression proteins, indirect labeling is often preferred to leverage secondary antibody signal amplification and increase sensitivity.
- In immunohistochemistry (IHC), indirect methods using secondary antibodies conjugated to enzymes (e.g., HRP, AP) are frequently applied because their strong amplification effect enables detection of trace antigens. Depending on the experimental needs, researchers must balance speed and convenience against signal intensity.
- In cell imaging, direct labeling is suitable for quickly observing protein localization and distribution, while indirect labeling is more effective for detecting low-abundance proteins or weak signals. Multiplex staining can also be combined for multichannel colocalization analysis.
Factors Influencing Labeling Strategy Selections
When choosing between direct and indirect labeling, several experimental factors must be considered:
- Sample Type and Target Abundance: Direct labeling is efficient for abundant antigens with minimal sensitivity requirements. For rare or low-expression targets, indirect labeling provides clear advantages through signal amplification.
- Multiplexing Requirements and Fluorophore Compatibility: In multicolor experiments, spectral overlap between fluorophores must be avoided. Selecting appropriate excitation/emission wavelengths and using cross-adsorbed secondary antibodies from the same host species helps minimize nonspecific binding.
- Desired Detection Sensitivity and Quantification Accuracy: Direct labeling generally provides a 1:1 quantitative relationship (one antibody to one signal), simplifying data analysis. Indirect labeling requires calibration to account for amplification to achieve accurate quantification.
- Instrumentation (Microscopy, Flow Cytometer, Plate Reader): Differences in excitation sources, filter configurations, and detector sensitivity require compatible fluorophore selection. Instruments may offer multiple UV, visible, or near-infrared excitation options, guiding dye choice.
- Optical Density and Quantum Yield of Fluorophores: Brightness (optical density and quantum yield) and photostability are important considerations. High-sensitivity or high-resolution imaging experiments favor bright, photostable dyes, while routine detection can use standard fluorophores.
Practical Considerations for Successful Labeling
To achieve stable and reliable fluorescent labeling, the following practical aspects should be addressed:
- Labeling Chemistry — NHS Ester, Maleimide, Click Reaction: NHS esters efficiently react with antibody amines, commonly performed in pH 8.0–8.5 buffers. Maleimides selectively target thiol groups (Cys residues), often requiring prior reduction of disulfide bonds with DTT or TCEP. Combinatorial strategies, such as SMCC linkers, allow dual-site labeling. Click chemistry (azide-alkyne cycloaddition) enables high-specificity, bioorthogonal labeling under mild conditions, suitable for cell or live-cell applications.
- Buffer Conditions, Purification, and Antibody Stability: NHS reactions are pH-sensitive; carbonate buffers or PBS (~pH 8.3) are recommended. Avoid free-amine buffers (e.g., Tris) that compete with NHS esters. Remove excess dye via dialysis, gel filtration, or ultrafiltration to reduce background. Conduct labeling at low temperature (4°C) and protect from light to preserve antibody stability and antigen-binding activity.
- Minimizing Background and Photobleaching: Block nonspecific binding using 5–10% normal serum (matched to secondary antibody host), BSA, skim milk, or gelatin. For indirect experiments, pre-blocking with normal serum of the same host species reduces secondary antibody binding to endogenous IgG. Minimize light exposure and use anti-fade mounting media to reduce photobleaching.
- Quality Control and Fluorescence Validation: Verify degree of labeling (DOL) by optical density measurements. Assess labeling purity via SDS-PAGE with fluorescence scanning. Functional assays like ELISA or flow cytometry confirm specificity and retained antibody activity.
BOC Sciences' Expertise in Fluorescent Antibody Labeling
BOC Sciences offers extensive experience and a comprehensive service platform for fluorescent antibody labeling, supporting research, diagnostic, and industrial applications. We provide customizable direct and indirect labeling services, including conjugating desired fluorophores to client-supplied primary antibodies or developing universal fluorescent secondary antibodies compatible with multiple primary antibodies. Our portfolio covers a wide range of reactive fluorophores and crosslinkers (various NHS esters, maleimide linkers, and azide/alkyne derivatives), supporting virtually all common fluorophore conjugation chemistries.
Custom Direct and Indirect Antibody Labeling Solutions
- Antibody Selection and Optimization: Choose the best antibody and optimize conjugation based on antigen properties, sample type, and application.
- Dye Design and Customization: Wide selection of fluorophores (FITC, Alexa Fluor, Cy series) with customization for specific excitation/emission requirements.
- Conjugation Strategy Design: Support NHS ester, maleimide, and click chemistry to maximize labeling efficiency while preserving antibody activity.
- Multifunctional Labeling: Enable multiplex and colocalization experiments for reliable analysis of complex samples.
Comprehensive Portfolio of Reactive Fluorophores and Crosslinkers
- Diverse Fluorophore Options: Includes FITC, Alexa Fluor, Cy series, BODIPY, covering blue to far-red spectra for multichannel and multiplex labeling.
- Various Reactive Groups: NHS ester for lysine labeling, maleimide for cysteine-selective conjugation, click chemistry for bioorthogonal reactions.
- Broad Compatibility: Fluorophores and linkers compatible with antibodies, proteins, nanomaterials, and biomolecules for covalent labeling and multifunctional applications.
- Customizable: Dye functional groups and conjugation ratios can be adjusted to achieve optimal fluorescence performance and signal stability.
Analytical Characterization and Performance Optimization
- Conjugation Efficiency Verification: HPLC, LC-MS, and SDS-PAGE to assess antibody labeling efficiency and purity.
- Fluorescence Performance Assessment: Measure fluorescence intensity and stability using microscopy, flow cytometry, or fluorometry.
- Batch Consistency Control: Ensure reproducible antibody labeling across batches.
- Signal Optimization: Adjust conjugation ratios and dye selection based on target abundance and experimental conditions to achieve high sensitivity with low background.
Scalable Production for Research, Diagnostic, and Commercial Use
- Research Grade: Suitable for small-scale laboratory assays and rapid experimental optimization.
- Diagnostic Grade: Meets clinical diagnostic reagent requirements for labeling consistency and reliability.
- Commercial Production: Supports large-scale antibody labeling with consistent performance and global distribution capability.
- Flexible Customization: Offers varied concentrations, packaging formats, and lyophilized or liquid forms according to customer needs.
Do You Need A Consultation?
BOC Sciences integrates cutting-edge fluorescence technologies to accelerate your research, driving next-generation solutions for drug discovery and diagnostics.
Transform Your Studies with Cutting-Edge Fluorescent Products
| Cat. No. | Name | CAS No. | Inquiry |
|---|---|---|---|
| F01-0012 | 3-Bodipy-propanoic acid | 165599-63-3 | Bulk Inquiry |
| F01-0064 | meso-CH2Br-BODIPY | 216434-81-0 | Bulk Inquiry |
| A16-0033 | 6-Carboxyfluorescein | 3301-79-9 | Bulk Inquiry |
| A16-0201 | DAPI dihydrochloride | 28718-90-3 | Bulk Inquiry |
| A15-0005 | 5(6)-Carboxyfluorescein | 72088-94-9 | Bulk Inquiry |
| F04-0033 | 5-Aminofluorescein | 3326-34-9 | Bulk Inquiry |
| F04-0034 | 5-Carboxyfluorescein diacetate | 79955-27-4 | Bulk Inquiry |
| F05-0031 | 6-Carboxy-X-rhodamine | 194785-18-7 | Bulk Inquiry |
| F01-0254 | BODIPY 493/503 carboxylic acid | 216961-95-4 | Bulk Inquiry |
| F03-0022 | Sulfo-Cyanine 5 Carboxylic Acid (ethyl) | 146368-11-8 | Bulk Inquiry |
High-Performance Fluorescent Tools for Your Research
- TAMRA Dyes Red-emitting dyes for antibody and protein labeling.
- ATTO Dyes High-performance dyes for labeling and imaging experiments.
- Coumarin Blue-emitting dyes for chemical sensing and fluorescence studies.
- Fluorescent Protein Used for live-cell imaging and real-time biosensing.
More About Antibody labeling
Online Inquiry

