Cytoskeleton Fluorescent Probes
-

-

-

-
 BF 350 Phalloidin
BF 350 PhalloidinCAT:
-
 BF 488 Phalloidin
BF 488 PhalloidinCAT:
-
 BF 568 Phalloidin
BF 568 PhalloidinCAT:
-

-

-

-
 Biotin-XX phalloidin
Biotin-XX phalloidinCAT:
-

Background
Fluorescent reagents, as essential tools in cell biology research, play an irreplaceable role in the visualization and analysis of the cytoskeleton. The cytoskeleton is composed of three main structural elements: actin filaments, microtubules, and intermediate filaments. It maintains cell morphology, participates in cell movement and division, and regulates intracellular signal transduction. By specifically binding fluorescent molecules to cytoskeletal proteins, researchers can achieve high-resolution imaging and dynamic observation of cytoskeletal structures under fluorescence microscopy. With the advancement of fluorescence technology, the variety of fluorescent dyes has become increasingly rich. Their broad application value is demonstrated in precise staining of different cytoskeletal components, multiplex labeling, and super-resolution imaging, greatly promoting in-depth research on cellular structure and function.
What is Cytoskeleton?
The cytoskeleton is a complex reticular structural system composed of proteins, located in the cytoplasm of eukaryotic cells. It is not only the supporting framework of the internal cell structure but also a core participant in the execution of cellular functions. The cytoskeleton is widely distributed within the cytoplasm and interacts with the cell membrane, organelles, and nuclear skeleton, collectively maintaining the structural integrity, shape, and spatial positioning of the cell. The cytoskeleton is not a static scaffold, but rather highly dynamic. Various signal transduction pathways regulate the polymerization state, localization, and interactions of cytoskeletal proteins, thereby influencing life processes such as the cell cycle, polarity, and differentiation. Therefore, the cytoskeleton has become a central subject of research in cell biology, developmental biology, oncology, and neuroscience.
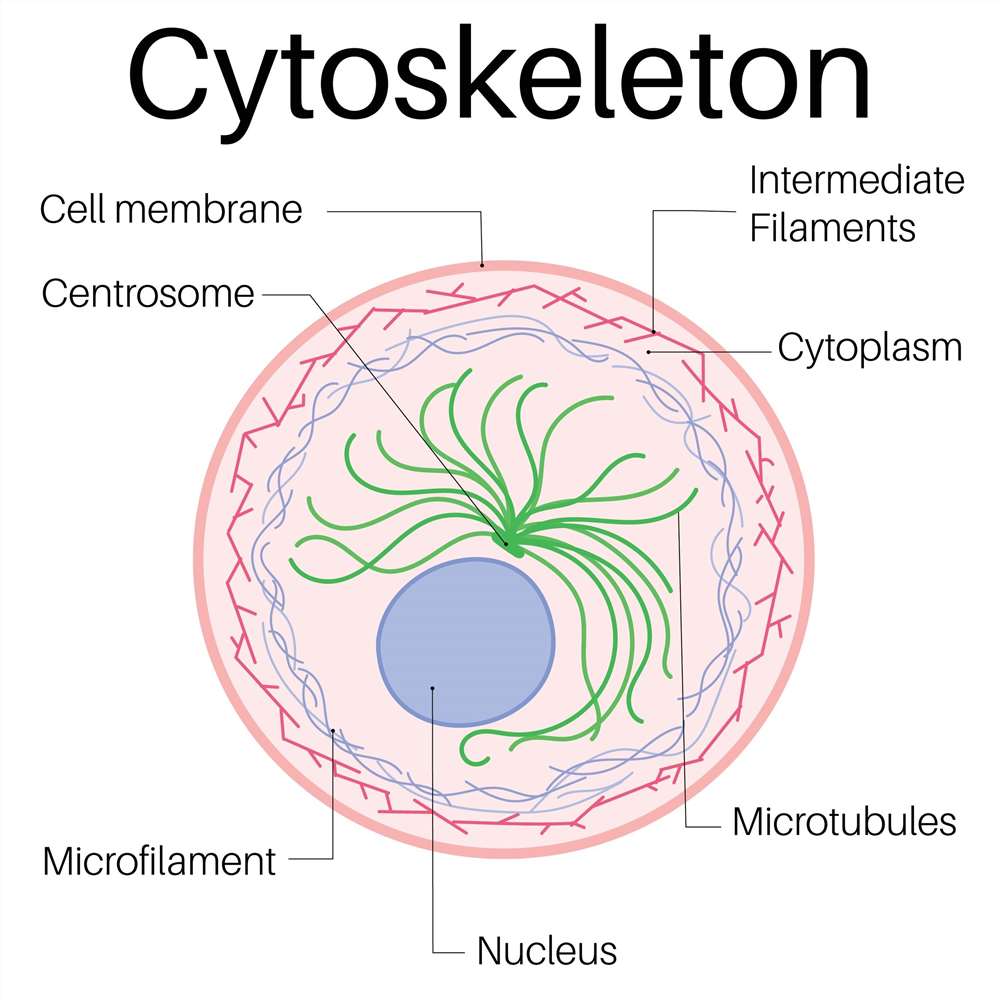 Fig. 1. Structure of cytoskeleton (BOC Sciences Authorized).
Fig. 1. Structure of cytoskeleton (BOC Sciences Authorized).
Cytoskeleton Structure and Function
The cytoskeleton is mainly composed of three types of fibrous structures: microfilaments (actin filaments), microtubules, and intermediate filaments. These three structures differ in diameter, protein composition, mechanical properties, and functions, yet they coordinate with one another to carry out various cellular activities.
Microfilaments
Microfilaments are primarily polymerized from actin monomers, with a diameter of approximately 7 nm, making them the thinnest fibers in the cytoskeleton. They are widely distributed in the cell cortex (areas near the plasma membrane) and play vital roles in maintaining cell shape, cell movement (such as crawling and phagocytosis), endocytosis and exocytosis, and cell division. Due to their rapid polymerization and depolymerization, microfilaments are critically important for the regulation of dynamic cellular behaviors.
Microtubules
Microtubules are hollow tubular structures formed by α- and β-tubulin dimers, with a diameter of about 25 nm, making them the thickest components of the cytoskeleton. Microtubules are organized from the centrosome and radiate toward the cell periphery. They are involved in chromosome separation, spindle formation during mitosis, organelle (such as mitochondria and endoplasmic reticulum) positioning, and vesicle transport, serving as the cell's "railway system." Many molecular motors, such as dynein and kinesin, travel along microtubules to conduct long-distance material transport.
Intermediate Filaments
Intermediate filaments have a diameter between that of microfilaments and microtubules, approximately 10 nm. Their protein composition varies depending on the cell type, including keratin, neurofilament proteins, vimentin, etc. The primary function of intermediate filaments is to enhance the mechanical strength of cells, enabling them to resist external mechanical stress. They are widely present in epithelial cells, neurons, and muscle cells, serving as key components for maintaining tissue structural stability.
Fluorescence Assay Principles
Fluorescent reagents are chemical substances capable of absorbing light energy at specific wavelengths and emitting fluorescence at another wavelength when returning from the excited state to the ground state. This light-emitting phenomenon is called fluorescence and is a crucial tool widely applied in modern cell biology and molecular imaging. In cytoskeletal staining, fluorescent reagents specifically label structural proteins of the cytoskeleton, allowing researchers to clearly visualize dynamic structures such as microfilaments, microtubules, and intermediate filaments under fluorescence microscopy and observe their distribution, rearrangement, and functional changes in real time. In cytoskeletal staining, fluorescent reagents should possess the following basic properties:
- High specificity: Able to accurately recognize and bind to target cytoskeletal proteins (such as actin, tubulin, or intermediate filament proteins) to avoid background staining and nonspecific signal interference.
- Excellent optical properties: Including high quantum yield (fluorescence efficiency), high photostability (resistance to photobleaching), and good Stokes shift, ensuring clear and durable imaging.
- Cell compatibility: In live-cell staining applications, fluorescent reagents must also exhibit low toxicity, good cell permeability, and stability to avoid interfering with physiological states of the cell.
Cytoskeleton Staining Fluorescent Reagents
In cytoskeletal staining, the selection of appropriate fluorescent reagents is critical for experimental success. Common fluorescent reagents include small-molecule fluorescent dyes, fluorescently labeled toxins, peptide probes, and antibodies or proteins conjugated with fluorophores. These reagents can be selectively used for different cytoskeletal components such as microfilaments (actin), microtubules (tubulin), and intermediate filaments (keratin, neurofilaments, etc.) to achieve high-resolution structural observation and functional studies.
Actin Cytoskeleton Staining
Phalloidin is a cyclic peptide extracted from Amanita phalloides that binds specifically and with high affinity to F-actin (filamentous actin) in a non-covalent manner. When conjugated with fluorophores (such as FITC, TRITC, or Alexa Fluor series), it forms fluorescently labeled phalloidin, which can be directly used for staining microfilaments in fixed cells. Its advantages include:
- High-specificity binding without disrupting actin dynamics;
- Clear staining patterns that accurately display cell edges, stress fibers, and pseudopodia structures;
- Availability in multiple fluorescence colors, allowing composite imaging with other markers.
Application example: In fibroblast or tumor cell studies, TRITC-phalloidin is commonly used to observe the formation and arrangement of stress fibers and analyze cell migration and adhesion behavior.
Microtubule Staining
Microtubule staining is commonly performed using two types of reagents:
- Anti-tubulin antibodies: Specific primary antibodies against α- or β-tubulin are used, followed by fluorescently labeled secondary antibodies (such as FITC- or Alexa Fluor 488-conjugated goat anti-rabbit IgG) for indirect labeling. This method is suitable for staining fixed cells or tissue sections, offering clear imaging and high sensitivity.
- Fluorescent Taxol derivatives: For example, Flutax-1 (FITC-Paclitaxel) can directly bind and stabilize microtubules, enabling real-time observation of microtubule network changes, especially useful in drug mechanism research.
Application example: Studying the arrangement of microtubules in neuronal axons or analyzing the effects of anti-microtubule drugs (e.g., paclitaxel) on microtubule dynamics.
Intermediate Filament Staining
Due to the diversity of intermediate filaments, staining strategies need to be selected based on the studied cell type and target protein. These antibodies are often conjugated with fluorophores such as Alexa Fluor, DyLight, Cy3, or Cy5, and are suitable for both single and multiplex labeling and imaging. Common labels include:
- Keratin antibodies (e.g., CK18, CK19): For labeling epithelial cells;
- Neurofilament protein antibodies: For staining neuronal structures;
- Vimentin antibodies: Often used for staining mesenchymal cells;
- Glial fibrillary acidic protein (GFAP) antibodies: For observing astrocyte cytoskeletons.
Application example: Observing intermediate filament remodeling during epithelial–mesenchymal transition (EMT) in tumor tissues using Cy3-labeled anti-keratin antibodies.
Cytoskeleton Staining Protocol and Steps
- Cell Fixation: Fix the cells with 4% paraformaldehyde at room temperature for 10–20 minutes to effectively crosslink intracellular proteins, preserving the in situ structure of the cytoskeleton and providing a stable morphological basis for subsequent staining.
- Cell Permeabilization: Treat the cells with 0.1% Triton X-100 for 5–10 minutes to disrupt membrane permeability, allowing fluorescent dyes or antibodies to enter the cell and bind sufficiently to cytoskeletal components.
- Blocking Non-specific Binding Sites: Block the cells with 1% bovine serum albumin (BSA) for 30 minutes to fill non-specific binding sites, preventing background staining by fluorescent reagents and improving labeling specificity and image clarity.
- Staining: According to the instructions of the selected fluorescent dye or antibody, add an appropriate amount of reagent and incubate in the dark for 30 minutes to 1 hour to allow binding to target cytoskeletal proteins.
- Washing: Gently wash with PBS buffer three times for 5 minutes each to remove free dyes thoroughly, reduce background interference, and ensure that imaging signals are authentic and clear.
- Imaging: Observe the sample using a fluorescence microscope or confocal microscope. Select appropriate filters based on the excitation and emission wavelengths of the dye to acquire high-quality images for analysis.
Application of Cytoskeleton Staining
Tumor Cell Migration Research
In studies of tumor cell invasion and metastasis, TRITC-Phalloidin is commonly used to stain F-actin, presenting the morphology and layout of pseudopodia and stress fibers at high resolution. By comparing differences in the length, density, and orientation of stress fibers between normal and metastatic cells, migration ability can be quantified. Combined with double staining using anti-α-/β-tubulin antibodies, the microtubule network and actin remodeling can be observed simultaneously, providing structural evidence for understanding cancer cell motility mechanisms.
Analysis of Neuronal Axon Growth
Alexa Fluor 488-labeled anti-β-tubulin antibodies are used to stain fixed cultured neurons, clearly visualizing the microtubule network in axons and dendrites. In conjunction with live-cell fluorescent Taxol probes such as Flutax-1, the dynamic polymerization and depolymerization of microtubules at the growth cone can be monitored in real time. This method provides rich morphological and dynamic information in studies of neural development, regeneration mechanisms, and degenerative disease models such as Alzheimer's disease.
Exploration of Drug Mechanisms of Action
After treating tumor cells with paclitaxel or colchicine, live-cell microtubules are stained in real time using FITC-Paclitaxel (Flutax-1), and observed with confocal microscopy for network changes. By quantifying microtubule bundling degree, breakage frequency, and length distribution, the half inhibitory concentration (IC₅₀) and expression changes of microtubule-associated proteins in resistant cell lines can be evaluated. This strategy provides intuitive and quantitative experimental evidence for elucidating the mechanism of action and optimization of combination therapies involving anti-microtubule drugs.
Epithelial–Mesenchymal Transition (EMT) Detection
During EMT, the expression profile of cytoskeletal proteins changes significantly: epithelial cells are rich in keratin, while mesenchymal cells predominantly express vimentin. In studies, Cy3-anti-CK18/CK19 and Alexa Fluor 488-anti-vimentin antibodies are used for dual staining of the same sample. Through multi-channel fluorescence imaging and post-acquisition image analysis, the fluorescence intensity and distribution ratio of the two markers can be precisely quantified, thereby monitoring the EMT process, which is of great significance in tumor metastasis and tissue remodeling research.
Multicolor Co-staining to Study Cytoskeletal Interactions
To comprehensively analyze spatial relationships among actin, microtubules, the nucleus, and mitochondria, a four-color co-staining approach is commonly used: Alexa Fluor 647-Phalloidin for F-actin, Alexa Fluor 488-anti-α-tubulin antibody for microtubules, DAPI for the nucleus, and MitoTracker Red for mitochondria. Multi-channel scanning using confocal microscopy allows direct visualization of the localization and interactions between the cytoskeleton and organelles within the same field of view, supporting studies in energy metabolism and signal transduction.
Resources

- Hoechst Dyes: Definition, Structure, Mechanism and Applications
- Mastering the Spectrum: A Comprehensive Guide to Cy3 and Cy5 Dyes
- Fluorescent Probes: Definition, Structure, Types and Application
- Fluorescent Dyes: Definition, Mechanism, Types and Application
- Coumarin Dyes: Definition, Structure, Benefits, Synthesis and Uses
- Unlocking the Power of Fluorescence Imaging: A Comprehensive Guide
- Cell Imaging: Definitions, Systems, Protocols, Dyes, and Applications
- Lipid Staining: Definition, Principles, Methods, Dyes, and Uses
- Flow Cytometry: Definition, Principles, Protocols, Dyes, and Uses
- Nucleic Acid Staining: Definition, Principles, Dyes, Procedures, and Uses
Online Inquiry

