Click Chemistry Ligands and Catalysts
Background
The history of Click Chemistry began in 2001, when the concept was first proposed by American chemist K. Barry Sharpless. Its core idea is to rapidly construct complex molecular structures through simple, efficient, and highly selective chemical reactions. The introduction of this concept marked a shift in chemical synthesis from complex multi-step reactions to simplified, efficient modular reactions. The emergence of click chemistry not only changed the way chemists think about synthesis but also brought revolutionary changes to numerous fields such as biological labeling, drug development, and materials science. Today, click chemistry has become an indispensable and important tool in modern chemical and biochemical research, continuously pushing the boundaries of science forward.
What is Click Chemistry?
Click chemistry is a chemical reaction concept characterized by simplicity, efficiency, and high selectivity. Compared to traditional chemical reactions, the core of click chemistry lies in its modularity, efficiency, and biocompatibility. Click reactions usually feature the following characteristics: mild reaction conditions without the need for harsh temperatures or pressures; fast reactions that can be completed in a short time; and products that are highly stable and biocompatible, posing no interference with biological systems. The core principle of click chemistry is "modularity." It emphasizes the simplicity and diversity of reaction substrates and connects different modules through simple chemical reactions to construct complex molecular structures. This modular approach makes chemical synthesis more efficient and flexible. For example, in drug development, researchers can use click chemistry to rapidly build large compound libraries, thereby accelerating the drug screening process.
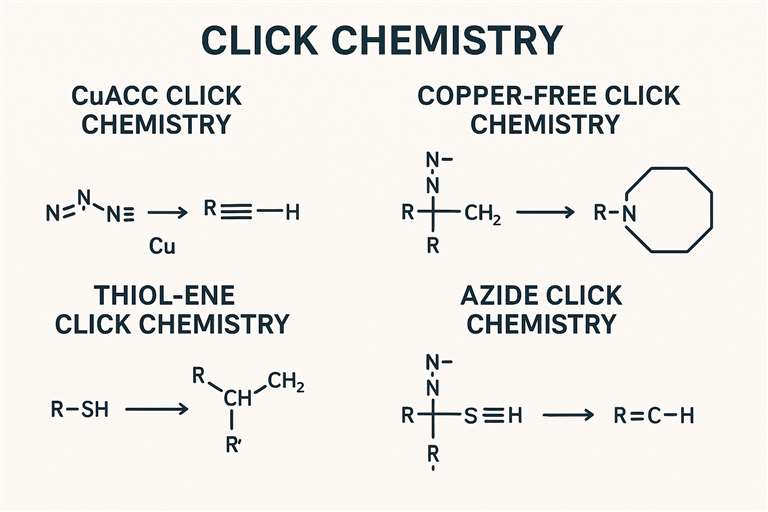 Fig. 1. Click reaction chemistry (BOC Sciences Authorized).
Fig. 1. Click reaction chemistry (BOC Sciences Authorized).
Click Chemistry Tools
With the widespread application of click chemistry, many commercial click chemistry reagents have emerged. These reagents include various click chemistry ligands, catalysts, and reagent kits. For example, BOC Sciences provides a wide range of click chemistry reagents, including azide groups, alkyne groups, thiols, and other ligands, as well as catalysts such as copper ions and radical initiators. These commercial reagents are of high quality and stability, capable of meeting researchers' needs under various experimental conditions.
Click Chemistry Ligands
Click chemistry ligands refer to molecular modules involved in click reactions. Common click chemistry ligands include azide groups (-N₃), alkyne groups (-C≡C-), and thiol groups (-SH). These ligands possess high reactivity and good biocompatibility, allowing them to undergo rapid click reactions with corresponding reactants. For example, azide groups can react with alkyne groups via CuAAC reactions or copper-free click reactions to form triazole ring products; thiols can undergo thiol-ene click reactions with alkenes to generate thioether products.
When selecting click chemistry ligands, researchers need to choose based on specific experimental needs and reaction conditions. For instance, if labeling needs to be performed in live cells, copper-free click ligands (such as DBCO) may be a better choice due to their high reactivity and biocompatibility under copper-free conditions. If labeling is required under mild conditions, thiol-ene click ligands may be preferable because their reaction conditions are mild and suitable for aqueous environments.
Click Chemistry Catalysts
Click chemistry catalysts refer to substances that can accelerate click reactions. Common click chemistry catalysts include copper ions and radical initiators. Copper ions are the key catalyst in CuAAC reactions; they can form stable complexes with azide and alkyne groups, thereby lowering the activation energy and accelerating the reaction. Radical initiators are the key catalysts in thiol-ene click reactions; they initiate the radical addition between thiols and alkenes, thereby speeding up the reaction.
When selecting click chemistry catalysts, researchers need to choose based on specific experimental needs and reaction conditions. For example, if labeling in live cells is required, copper-free click chemistry may be the better option since it does not involve copper ions, offering higher biocompatibility and safety. If labeling under mild conditions is required, radical initiators may be preferable because they support reactions in aqueous conditions under mild temperatures.
Types of Click Chemistry Reactions
CuAAC Click Chemistry
Copper-catalyzed azide-alkyne cycloaddition (CuAAC) is one of the most classic and widely used reactions in click chemistry. The core of this reaction is the cycloaddition between azide groups (-N₃) and alkyne groups (-C≡C-) catalyzed by copper ions to produce triazole ring-containing products. Copper ions play a key catalytic role by forming stable complexes with the azide and alkyne groups, reducing the activation energy and accelerating the reaction. CuAAC reactions are known for their high selectivity and fast reaction rates. In biological systems, the reaction has good biocompatibility and does not interfere with the function of biomolecules. Therefore, CuAAC reactions are widely applied in biomolecular labeling and surface modification of biomaterials. For example, researchers can use CuAAC reactions to connect fluorescent probes or drug molecules to biomolecules for real-time imaging or drug delivery.
Copper-Free Click Chemistry
Although CuAAC reactions offer many advantages, the presence of copper ions can be toxic to certain biological systems. Moreover, in some biomedical applications, copper ions may interfere with the function of biomolecules. To address this issue, researchers have developed copper-free click reactions. The core of copper-free click chemistry lies in using strain energy to drive the reaction, thereby avoiding the need for copper ions. In copper-free click chemistry, researchers typically use strained alkynes or azides as substrates. These strained substrates have high reactivity and can react with corresponding reactants under mild conditions. For example, DIBO (dibenzocyclooctyne) and DBCO (dibenzocyclooctyne azide) are two commonly used strained alkyne substrates that can undergo rapid click reactions with azide groups under copper-free conditions.
Thiol-Ene Click Chemistry
Thiol-ene click chemistry is a click reaction based on radical addition. The core of this reaction involves the addition between thiol groups (-SH) and alkenes (-CH=CH-) under the initiation of radical initiators, forming thioether products. The advantages of thiol-ene click chemistry include its mild reaction conditions, compatibility with aqueous media, and relatively fast reaction rates. Thiol-ene click chemistry is widely applied in surface modification of biomaterials and the development of biosensors. For example, researchers can use thiol-ene click chemistry to immobilize biomolecules or functional groups onto biomaterial surfaces, thereby imparting specific bioactivities. Additionally, thiol-ene click chemistry can be used to develop new biosensors by linking fluorescent or electrochemical probes to biomolecules, enabling highly sensitive detection of biomolecules.
Azide Click Chemistry
Azide click chemistry refers to click reactions centered around azide groups (-N₃). Besides the aforementioned CuAAC and copper-free click reactions involving azides, azide groups can also react with many other functional groups, such as alkynes, alkenes, and aldehydes. Azide groups exhibit high reactivity and good biocompatibility, making azide click chemistry highly promising for biomedical applications. For example, in drug development, researchers can use azide click chemistry to link drug molecules with biomolecules to achieve targeted drug delivery. In addition, azide click chemistry can be applied to the development of new biosensors and fluorescent probes by connecting azide groups with fluorescent dyes or electrochemical probes for highly sensitive detection of biomolecules.
Click Chemistry Mechanism
The core of click chemistry lies in rapidly connecting different molecular modules through simple chemical reactions, while maintaining high efficiency and biocompatibility. Its mechanism is mainly reflected in the following aspects:
Modular Design of the Reaction
Click chemistry emphasizes modularity in reactions, where the substrates are usually simple and easily synthesized molecular modules. These modules are connected via specific chemical reactions to form complex molecular structures. For example, azide groups (–N₃) and alkyne groups (–C≡C–) are among the most common modules in click chemistry. Through copper-catalyzed azide-alkyne cycloaddition (CuAAC), these two modules can rapidly react under mild conditions to produce stable triazole ring structures. This modular design not only simplifies the reaction steps but also enhances the controllability and efficiency of the reactions.
High Efficiency of the Reaction
Click chemistry reactions are typically characterized by extremely high reaction rates and selectivity. Taking CuAAC as an example, copper ions act as catalysts that significantly reduce the activation energy and accelerate the reaction. Copper ions form stable complexes with azide and alkyne groups, and through coordination, promote the proximity and reaction of the substrates. This highly efficient reaction mechanism allows click chemistry to construct complex molecules within a short time, making it suitable for high-throughput screening and large-scale synthesis.
Biocompatibility
Another important feature of click chemistry reactions is their biocompatibility. Many click chemistry reactions can proceed smoothly in biological systems without interfering with the functions of biomolecules. For instance, copper-free click chemistry uses strain energy to drive the reaction, avoiding the use of copper ions and thereby reducing toxicity to biological systems. This biocompatibility gives click chemistry broad application prospects in biomedicine, such as live-cell labeling, drug delivery, and biosensor development.
Universality of the Reaction
Click chemistry reactions are highly universal and compatible with various molecular modules. For example, azide groups can react not only with alkynes but also with other functional groups such as alkenes and aldehydes. This universality enables click chemistry to be applied in diverse chemical and biological systems, meeting various research and application demands.
Reversibility of the Reaction
Although most click chemistry reactions are irreversible, under specific conditions, some click reactions can be designed to be reversible. For example, by adjusting reaction conditions or introducing specific catalysts, certain click reactions can achieve dynamic equilibrium. This reversibility offers possibilities for studying dynamic processes in biological systems. For example, in investigating protein interactions or cellular signal transduction, reversible click chemistry reactions can be used to monitor and regulate biomolecular dynamics in real time.
Click Chemistry Examples
Click chemistry, known for its high efficiency, selectivity, and mild reaction conditions, has been widely applied in biolabeling, drug development, and materials science. The table below summarizes typical applications of click chemistry in systems such as biotin, antibodies, nucleic acids, and polymers. It highlights common reaction types like CuAAC, SPAAC, and thiol-ene, along with representative examples that demonstrate the versatility of click chemistry in modern research and industry.
| Application Category | Description | Common Click Reaction Types | Typical Uses or Examples |
| Biotin Click Chemistry | Biotin labeling via click chemistry for high-affinity capture and detection. | CuAAC, SPAAC | Protein labeling, probe construction, streptavidin-biotin purification and imaging. |
| Carbohydrate Click Chemistry | Modification of carbohydrates for labeling, glyco-conjugates, or vaccine development. | CuAAC, SPAAC, Staudinger ligation | Glycan microarrays, carbohydrate-based vaccines, targeted delivery systems. |
| Click Chemistry ADC | Synthesis of antibody-drug conjugates with stable and controllable linkers. | CuAAC, SPAAC, IEDDA | Site-specific antibody modification, drug-linker conjugation for enhanced therapies. |
| Click Chemistry Amino Acids | Functionalization of natural or non-natural amino acids. | CuAAC, SPAAC, Thiol-yne | Amino acid labeling, fluorescent probes, site-selective modification. |
| Click Chemistry Antibody | Linking antibodies to drugs, dyes, or other molecules via click reactions. | CuAAC, SPAAC, Tetrazine–TCO | High-purity antibody conjugates, immunoimaging, targeted therapy development. |
| Click Chemistry DNA | Chemical modification of DNA sequences for probe design or functional labeling. | CuAAC, SPAAC, Tetrazine–TCO | Fluorescent labeling, oligonucleotide drug development, DNA microarray construction. |
| Click Chemistry Polymer | Functionalization of polymer backbones or side chains for smart materials or biomedical use. | CuAAC, Thiol-ene, Diels–Alder | Functional polymers, hydrogels, targeted drug delivery systems. |
| PEG Click Chemistry | PEGylation of proteins, peptides, or drugs to improve solubility and stability. | CuAAC, SPAAC, Thiol–ene, Diels–Alder | Drug PEGylation, protein modification, long-circulating delivery vehicles. |
| Peptide Click Chemistry | Modification and cyclization of peptides for enhanced targeting, stability, or signal amplification. | CuAAC, Thiol–ene, Oxime ligation | Peptide probes, targeted delivery systems, cyclic peptide synthesis. |
| Protein Click Chemistry | Site-specific protein modification, often via unnatural amino acid incorporation. | CuAAC, SPAAC, Staudinger, Thiol–ene | Protein labeling, fusion protein construction, immunoassay reagent development. |
| RNA Click Chemistry | RNA modification to study structure-function relationships or build mRNA-based probes or vaccines. | CuAAC, SPAAC, Tetrazine–TCO | RNA labeling, synthetic mRNA modification, RNA-based diagnostic platforms. |
Applications of Click Chemistry
Bioorthogonal Click Chemistry
Bioorthogonal chemistry refers to chemical reactions that do not interfere with endogenous biochemical reactions within biological systems. Click chemistry is a vital component of bioorthogonal chemistry, offering excellent biocompatibility and selectivity in biological contexts without disrupting the function of biomolecules. For example, both CuAAC and copper-free click reactions can proceed smoothly in live cells without affecting normal physiological functions. This makes click chemistry widely applicable in bioorthogonal fields such as live-cell imaging, drug delivery, and surface modification of biomaterials.
Click Chemistry Bioconjugation
Bioconjugation refers to the process of linking biomolecules with other molecules (such as drug molecules or fluorescent probes). Click chemistry offers unique advantages in bioconjugation, allowing biomolecules to be linked with other molecules through simple reactions under mild conditions without damaging their function. For example, researchers can use click chemistry to link drug molecules to antibodies, thereby enabling targeted drug delivery. Furthermore, click chemistry can be used to develop novel biosensors and fluorescent probes by connecting biomolecules to fluorescent dyes or electrochemical probes, achieving highly sensitive biomolecular detection.
Click Chemistry Fluorescence Labeling
Fluorescence labeling involves linking fluorescent probes to biomolecules. Click chemistry has wide applications in fluorescence labeling, enabling the connection of fluorescent probes to biomolecules through simple reactions under mild conditions that preserve biomolecular function. For example, researchers can use click chemistry to label proteins, nucleic acids, and other biomolecules with fluorescent probes for real-time imaging. Additionally, click chemistry can be used to develop new types of fluorescent probes by connecting fluorescent dyes to biomolecules for highly sensitive detection.
Click Chemistry in Drug Discovery
Click chemistry plays a significant role in drug development. It allows for the rapid construction of large compound libraries by connecting different molecular modules through simple reactions. This enables researchers to quickly screen for compounds with potential pharmaceutical value and accelerate drug development. Moreover, click chemistry can be used to develop novel drug delivery systems by linking drug molecules with biomolecules to achieve targeted drug delivery. For example, researchers can use click chemistry to link drug molecules with antibodies to enhance drug efficacy and safety.
Click Chemistry in Materials Science
Click chemistry is also widely used in materials science. It allows the construction of functional materials by connecting different molecular modules through simple chemical reactions. For example, researchers can use click chemistry to attach biomolecules to material surfaces, imparting specific bioactivities to the materials. Additionally, click chemistry can be used to develop novel biomaterials and nanomaterials by linking various molecular modules to build materials with specific functions. For instance, researchers can use click chemistry to attach fluorescent probes to nanomaterial surfaces for real-time imaging and biomedical applications.
Conclusion
As an emerging chemical technology, click chemistry demonstrates great application potential in chemical synthesis and biological labeling due to its simplicity, high efficiency, and high selectivity. From copper-catalyzed azide-alkyne cycloaddition to copper-free click chemistry and thiol-ene click chemistry, the diverse reaction types of click chemistry offer flexible options for different application scenarios. Its broad applications in bioorthogonal chemistry, bioconjugation, fluorescence labeling, drug development, and materials science not only promote the integration of chemistry and biomedicine but also provide new ideas and tools for future scientific research and technological innovation.
Resources

- Hoechst Dyes: Definition, Structure, Mechanism and Applications
- Mastering the Spectrum: A Comprehensive Guide to Cy3 and Cy5 Dyes
- Fluorescent Probes: Definition, Structure, Types and Application
- Fluorescent Dyes: Definition, Mechanism, Types and Application
- Coumarin Dyes: Definition, Structure, Benefits, Synthesis and Uses
- Unlocking the Power of Fluorescence Imaging: A Comprehensive Guide
- Cell Imaging: Definitions, Systems, Protocols, Dyes, and Applications
- Lipid Staining: Definition, Principles, Methods, Dyes, and Uses
- Flow Cytometry: Definition, Principles, Protocols, Dyes, and Uses
- Nucleic Acid Staining: Definition, Principles, Dyes, Procedures, and Uses
Online Inquiry





