Quencher
Customized Fluorescent Reagents
One-stop Solution for Your Research
BOC Sciences offers a one-stop solution for fluorescent reagents, providing custom synthesis, modification, and large-scale production services. Our comprehensive portfolio includes high-purity fluorescent dyes, probes, and labeling reagents for research and industrial applications, ensuring superior performance and reliability.
Explore More

Background
Fluorescence technology serves as an essential optical analysis technique that remains indispensable across biochemistry, materials science, environmental monitoring, and medical diagnostics. Fluorescent molecules take in light at particular wavelengths and emit light at longer wavelengths through the process of fluorescence. External factors frequently diminish fluorescence signals in practical applications through a process called fluorescence quenching. Fluorescence quenching represents both an obstacle that requires resolution and a valuable instrument for scientific analysis. The examination of fluorescence quenching enables scientists to understand molecular interactions more deeply while creating advanced sensors and exploring complex biological systems.
What is Fluorescence Quenching?
Fluorescence quenching describes the reduction in fluorescence intensity or lifetime of a molecule which occurs when it interacts with external quenchers during its excited state. The quenching process operates through several pathways which include energy transfer along with electron transfer and collisional quenching. The quenching substance disrupts normal fluorescence emission by interacting with fluorophores. The action mechanisms of quenchers are mainly categorized into static quenching and dynamic quenching: Static quenching happens through the formation of a non-fluorescent complex between the quencher and the ground-state fluorophore while dynamic quenching stems from collisions between the quencher and the excited-state fluorophore that result in energy transfer or non-radiative transitions. Potassium iodide and oxygen together with heavy metal ions represent typical quenching agents. These applications extend across multiple domains including biomedicine and environmental monitoring and materials science where they help detect biomolecular interactions monitor oxygen levels and create new fluorescent sensor technologies.
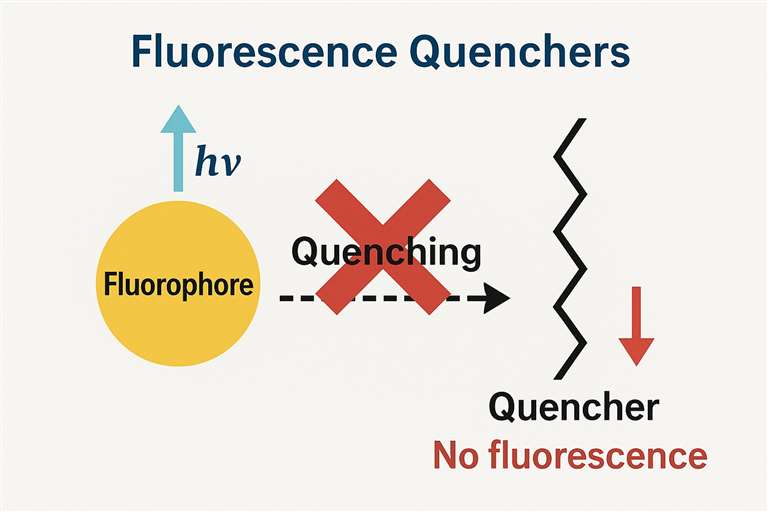 Fig. 1. Fluorescence quenching (BOC Sciences Authorized).
Fig. 1. Fluorescence quenching (BOC Sciences Authorized).
How Does Fluorescence Quenching Work?
In the process of fluorescence quenching, changes in fluorescence lifetime are also an important parameter. Fluorescence lifetime refers to the average time a fluorescent molecule remains in the excited state before returning to the ground state. During fluorescence quenching, the fluorescence lifetime may be shortened, which can be measured by time-resolved fluorescence spectroscopy. Changes in fluorescence lifetime provide more information about quenching mechanisms and molecular interactions. According to different mechanisms, fluorescence quenching mainly includes the following types:
Static Fluorescence Quenching
Static quenching is a type of fluorescence quenching in which the quencher forms a non-fluorescent complex with the fluorophore in the ground state. This quenching process is usually associated with chemical reactions or physical adsorption. For example, iodide ions (I⁻) in potassium iodide (KI) can interact electrostatically with fluorescent molecules, altering their electronic structure and thereby suppressing fluorescence emission. A notable feature of static quenching is that fluorescence intensity can significantly decrease even at very low quencher concentrations. This is because the formation of non-fluorescent complexes is a chemical equilibrium process—once such complexes form, the fluorophore can no longer emit fluorescence.
Dynamic Quenching in Fluorescence
Dynamic quenching is another type of fluorescence quenching that occurs when the excited-state fluorophore collides with the quencher, resulting in fluorescence quenching. This process is reversible—the fluorophore can recover its fluorescence properties in the absence of the quencher. The efficiency of dynamic quenching depends on the quencher concentration, the diffusion coefficient of the fluorophore, and the strength of their interactions. For instance, oxygen is a common dynamic quencher that can collide with the excited-state fluorophore and transfer its energy to the oxygen molecule, causing fluorescence quenching. A key feature of dynamic quenching is that fluorescence intensity decreases proportionally with quencher concentration, and fluorescence lifetime also shortens.
Fluorescence Self-Quenching
Self-quenching refers to the fluorescence quenching phenomenon that occurs when fluorescent molecules interact with each other at high concentrations. This typically results from molecular interactions among the fluorescent molecules themselves. For example, fluorescent dyes may aggregate at high concentrations, suppressing fluorescence emission. A notable feature of self-quenching is that fluorescence intensity decreases as the concentration of the fluorescent molecules increases. This phenomenon must be carefully considered in the design of fluorescent probes, as excessively high dye concentrations may weaken the fluorescence signal.
Intrinsic Fluorescence Quenching
Intrinsic quenching refers to the reduction of fluorescence signals due to specific groups or elements within the fluorophore's own structure. Common structures include aromatic side chains, thiol (-SH) groups, carboxyl groups, and certain metal ion coordination sites. These structures can provide non-radiative energy dissipation pathways, affecting the relaxation of excited-state electrons. For example, tryptophan residues in proteins may undergo spontaneous fluorescence quenching due to environmental changes, reflecting their conformational state.
Oxygen Fluorescence Quenching
Oxygen molecules are highly efficient triplet-state quenchers that can undergo energy transfer reactions with excited-state fluorophores, leading to non-radiative relaxation and reduced fluorescence intensity. This quenching process is directly related to oxygen concentration and is thus widely used in the development of oxygen sensors, hypoxia detection in biological tissues, and environmental oxygen monitoring devices. It holds particular application value in medical diagnostics and food packaging monitoring.
Heavy Atom Effect Fluorescence Quenching
Heavy atoms (e.g., iodine, bromine, lead) can enhance spin-orbit coupling in molecules, promoting intersystem crossing of excited-state electrons and accelerating energy dissipation, leading to fluorescence quenching. This effect plays a key role in photosensitizer design, fluorescent switch construction, and regulation of fluorescence material performance. It is a common strategy for tuning fluorescence lifetime and quantum yield.
Fluorescence Quenchers
A fluorescence quencher is a type of chemical substance that interacts with fluorophores and reduces fluorescence emission intensity through various mechanisms. Quenchers do not necessarily chemically degrade the fluorophore but act through non-radiative processes such as energy transfer, electron transfer, or excited-state interference to diminish or eliminate the fluorescence signal. The quenching process can be reversible (e.g., collisional quenching) or irreversible (e.g., quenching caused by certain chemical reactions). The degree of quenching is closely related to quencher concentration, temperature, molecular diffusion rate, and the solution environment.
Chemical Quenchers
Chemical quenchers are substances that interact with fluorescent molecules via chemical reactions or physical adsorption. Common chemical quenchers include potassium iodide (KI), ammonium acetate (NH₄Ac), etc. These quenchers can inhibit fluorescence emission by altering the electronic structure of fluorophores. For instance, iodide ions in potassium iodide can quench fluorescence through electrostatic interaction with fluorescent molecules.
Biological Quenchers
Biological quenchers are biomacromolecules capable of interacting with fluorescent molecules. For example, proteins can bind to fluorophores and alter their fluorescence properties. This quenching process is usually closely related to the structure and function of biomolecules. For instance, in the study of protein-ligand interactions, fluorescence quenching serves as an effective tool to investigate binding constants and kinetic parameters by monitoring fluorescence intensity changes.
Gaseous Quenchers
Gaseous quenchers are gas molecules that quench fluorescence by colliding with fluorescent molecules. Oxygen is a common gaseous quencher that interacts with excited-state fluorophores and transfers energy to the oxygen molecule, causing fluorescence quenching. Oxygen quenching is a dynamic quenching process, with efficiency directly proportional to oxygen concentration. This phenomenon is significant in biological systems—for example, changes in intracellular oxygen concentration can be monitored by fluorescence quenching sensors.
Fluorescence Quencher List
According to the quenching mechanisms, common quenchers include molecular oxygen, iodide ions, metal ions, quinone compounds, as well as synthetically designed black hole quenchers (BHQ series) and DABCYL. Different types of quenchers exhibit unique characteristics in selectivity, stability, quenching efficiency, and spectral properties, thus meeting the needs of various experimental systems. Mastery of the types and mechanisms of common fluorescence quenchers is essential for efficiently designing fluorescence labeling systems and enhancing signal sensitivity and specificity.
| Type | Quenching Mechanism | Representative Compounds | Features and Applications |
| Molecular Oxygen (O₂) | Dynamic Quenching | Oxygen | Naturally present; used to study excited-state lifetimes and oxygen-sensitive systems. |
| Iodide Ion (I⁻) | Dynamic Quenching | KI | High-efficiency collisional quencher; useful in protein structure probing. |
| Metal Ions | Static/Dynamic Quenching | Cu²⁺, Fe³⁺, Hg²⁺ | Coordinate with fluorophores; commonly used in ion sensors. |
| Quinone Compounds | Electron Transfer | Benzoquinone, Quinonimine | Conjugated structures; useful in small molecule detection. |
| Black Hole Quenchers (BHQ) | FRET Quenching | BHQ-1, BHQ-2, BHQ-3 | Non-fluorescent, broad-spectrum; widely used in qPCR and molecular probes. |
| DABCYL | FRET Quenching | 4-(Dimethylaminoazo)benzene | Classical FRET acceptor; commonly used in DNA/RNA probe systems. |
| DusQ | FRET Quenching | DusQ series | Commercial quenchers with low background signal; ideal for multiplex detection. |
| QSY | FRET Quenching | QSY-7 , QSY-21 , etc. | High-efficiency, broad-spectrum quenchers; suitable for real-time probes and imaging systems. |
| Carbonyl Compounds | Triplet-State Quenching | Aromatic aldehydes, pyridinecarboxaldehydes | Induce non-radiative triplet-state deactivation; used in specific mechanistic studies. |
Fluorescence Quenching Assay
The basic steps of a fluorescence quenching assay include the selection of a fluorescent probe, the addition of a quencher, the measurement of fluorescence intensity, and data analysis. The selection of a fluorescent probe is one of the critical steps in the experiment. The probe should have a high fluorescence quantum yield, good photostability, and specificity. The quencher must be selected based on the experimental objective. For example, if studying oxygen quenching, oxygen should be used as the quencher. Fluorescence intensity is usually measured using a fluorescence spectrometer. By measuring changes in fluorescence intensity, the interaction between fluorescent molecules and quenchers can be understood.
Fluorescence Quenching Analysis
Data analysis in fluorescence quenching experiments is typically based on the Stern-Volmer equation. The Stern-Volmer equation describes the relationship between fluorescence intensity and quencher concentration. By measuring the fluorescence intensity at different quencher concentrations, quenching constants and binding constants can be calculated. The quenching constant reflects the interaction strength between fluorescent molecules and quenchers, while the binding constant reflects their binding affinity. Analysis using the Stern-Volmer equation provides insight into the quenching mechanism and the nature of intermolecular interactions.
Application of Fluorescence Quenching
As a sensitive and quantifiable phenomenon, fluorescence quenching plays a vital role in numerous scientific research areas and practical applications. Due to its high sensitivity to environmental changes—such as intermolecular distance, quencher concentration in solution, and oxygen content—fluorescence quenching serves as an essential tool for studying molecular interactions, detecting target substances, and constructing biosensors.
Study of Intermolecular Interactions
By monitoring changes in fluorescence intensity, researchers can evaluate the binding affinity, number of binding sites, and kinetic parameters between fluorophores and other molecules. Interactions among biomacromolecules, such as protein-ligand, antibody-antigen, and nucleic acid hybridization, are commonly elucidated via static or dynamic quenching mechanisms. This is widely applied in drug screening, structural biology, and biomolecular engineering.
Oxygen Sensing and Biomedical Detection
Oxygen is a common quencher, and its quenching capability has been applied to the development of oxygen sensors and tissue oxygen partial pressure monitoring devices. By quantitatively analyzing changes in fluorescence lifetime or intensity, real-time detection of key physiological states such as cellular respiration, tissue hypoxia, and tumor microenvironments can be achieved. Furthermore, similar principles are used in the design of oxygen sensing systems for food preservation, industrial fermentation, and environmental monitoring.
Fluorescent Probes and Imaging Technologies
In cellular and tissue imaging, designing fluorescent probes with quenchable and recoverable properties enables high-contrast and high-selectivity biological labeling. For instance, Förster Resonance Energy Transfer (FRET) technology is based on fluorescence quenching due to energy transfer between a donor and acceptor, and is widely used to study molecular conformational changes, enzymatic reactions, and cell signaling pathways.
Drug Delivery and Release Monitoring
Fluorescence quenching is employed to construct intelligent drug release systems. When drug-loaded nanoparticles enter cells or specific environments, the quenched state is reversed, restoring the fluorescence signal and indicating drug release. This mechanism enhances drug targeting and therapeutic efficacy and is one of the key strategies in the design of nanomedicine systems.
Analytical Chemistry and Environmental Monitoring
In the detection of heavy metals and organic pollutants, certain contaminants can act as quenchers and interact with fluorescent probes, enabling highly sensitive detection. The quenching effect offers a simple and non-destructive method, widely used in water quality analysis, food safety, and industrial process monitoring.
Conclusion
As a significant optical phenomenon, fluorescence quenching has broad application prospects in both scientific research and practical fields. This article introduces the definition, principles, types, common quenchers, applications, and experimental methods of fluorescence quenching in detail, and discusses the challenges and future development directions. Fluorescence quenching can not only be used as a tool to study molecular interactions but also for developing high-sensitivity sensors. With the continuous advancement of science and technology, fluorescence quenching techniques will play an increasingly important role in biomedicine, environmental monitoring, and materials science.
Resources

- Hoechst Dyes: Definition, Structure, Mechanism and Applications
- Mastering the Spectrum: A Comprehensive Guide to Cy3 and Cy5 Dyes
- Fluorescent Probes: Definition, Structure, Types and Application
- Fluorescent Dyes: Definition, Mechanism, Types and Application
- Coumarin Dyes: Definition, Structure, Benefits, Synthesis and Uses
- Unlocking the Power of Fluorescence Imaging: A Comprehensive Guide
- Cell Imaging: Definitions, Systems, Protocols, Dyes, and Applications
- Lipid Staining: Definition, Principles, Methods, Dyes, and Uses
- Flow Cytometry: Definition, Principles, Protocols, Dyes, and Uses
- Nucleic Acid Staining: Definition, Principles, Dyes, Procedures, and Uses
Online Inquiry

