Black Hole Quencher (BHQ)
-

-

-

-

-

-

-

-

-

-

-

-

-
 3'-BHQ-3 CPG
3'-BHQ-3 CPGCAT:
Background
Fluorescence technology is an indispensable tool in modern biology, medical diagnostics, and materials science. In techniques such as molecular probes, real-time PCR, fluorescence resonance energy transfer (FRET), and bioimaging, the excitation and emission properties of fluorescent dyes are central to signal detection. However, in these applications, controlling fluorescence signal intensity, improving signal specificity, and reducing background interference have become key goals for researchers. To address these challenges, fluorescence quenchers have emerged. Among numerous quenchers, black hole quenchers (BHQ) stand out due to their ability to efficiently absorb energy without emitting fluorescence. They are widely used in molecular probes, fluorescent probe design, and nucleic acid detection systems.
What is a Black Hole Quencher?
Black hole quenchers (BHQ) are a class of highly efficient fluorescence quenching molecules widely used in the design of fluorescent probes in molecular biology, especially in real-time quantitative PCR (qPCR) and FRET techniques. BHQs do not emit fluorescence themselves but quench fluorescence by absorbing the energy emitted by fluorescent dyes through non-radiative energy dissipation. This effectively reduces background signals and improves detection sensitivity. Depending on their absorption wavelength ranges, BHQs are classified into several types such as BHQ-0, BHQ-1, BHQ-2, and BHQ-3, which can be paired with fluorescent dyes of different wavelengths to support multiplex fluorescence detection experiments.
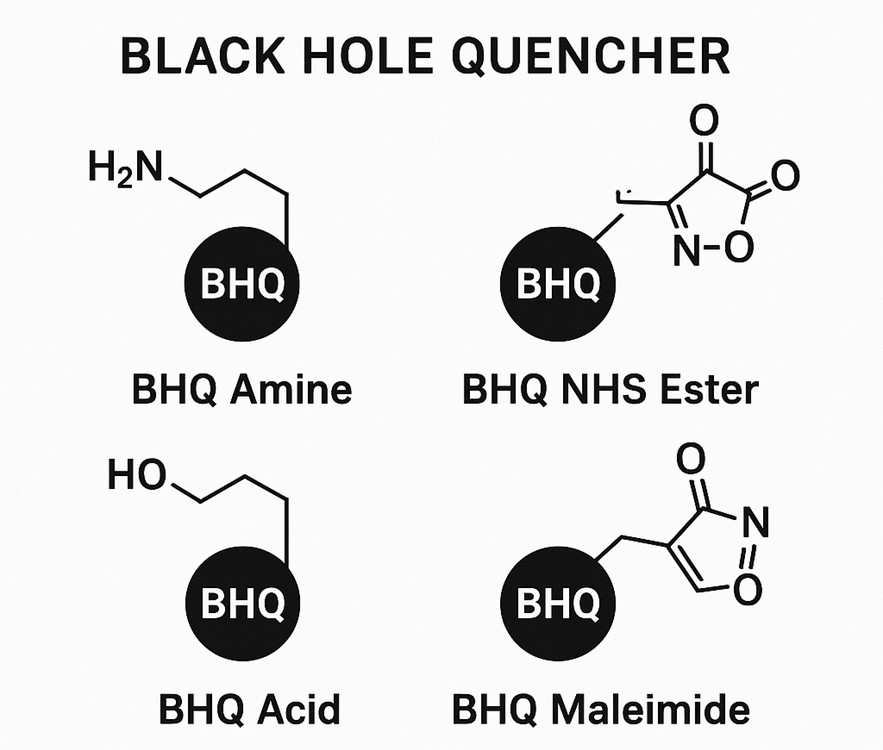 Fig. 1. BHQ quenchers (BOC Sciences Authorized).
Fig. 1. BHQ quenchers (BOC Sciences Authorized).
BHQs exhibit numerous significant advantages in fluorescence detection technologies, making them an ideal choice for molecular diagnostics and life science research. First, BHQs do not possess intrinsic fluorescence emission, which means they do not generate background fluorescence during detection, significantly enhancing overall detection sensitivity—especially suitable for identifying and analyzing low-concentration target molecules. Second, the BHQ dye family covers a broad spectral absorption range, extending from the visible to near-infrared regions, allowing for flexible pairing with various fluorescent dyes. Lastly, compared to traditional quenchers such as TAMRA, BHQs produce virtually no background interference and do not emit weak fluorescence, greatly improving signal accuracy and detection reliability. These advantages make BHQs irreplaceable in high-sensitivity, low-background, and multi-channel fluorescence detection experiments.
Black Hole Quencher Structure
The structural design of BHQs is sophisticated and unique. They are based on multi-aromatic azo backbones. By introducing electron-donating and electron-withdrawing groups onto the aromatic rings, a series of quenchers with broad absorption curves is produced. This structural design enables BHQ dyes to absorb light over a wide wavelength range, effectively quenching the emission of various fluorescent dyes. Unlike traditional fluorescent quenchers (e.g., TAMRA), BHQ dyes themselves are non-fluorescent. This feature is crucial, as it avoids background fluorescence interference and significantly improves the signal-to-noise ratio in fluorescence detection. Background fluorescence is often a critical factor affecting detection sensitivity and accuracy. The non-fluorescent property of BHQ dyes makes them an ideal choice in fluorescence detection.
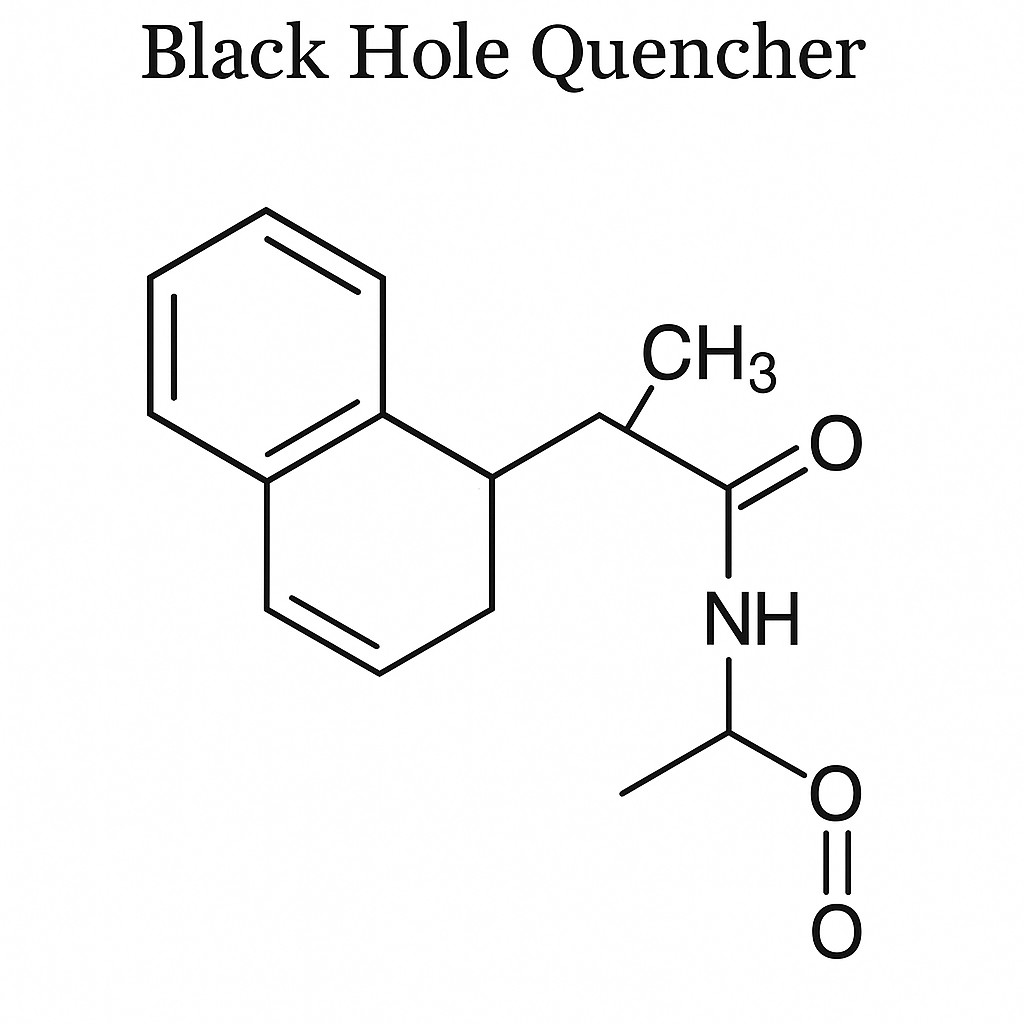 Fig. 2. The structural of BHQs (BOC Sciences Authorized).
Fig. 2. The structural of BHQs (BOC Sciences Authorized).
Black Hole Quencher Spectrum
The BHQ dye family comprises several members classified by their absorption wavelengths, with common types including BHQ-1, BHQ-2, and BHQ-3.
This broad absorption range enables BHQ dyes to be perfectly matched with various fluorescent dyes to meet different experimental needs. Their absorption ranges are as follows:
| Type | Absorption Wavelength Range | Compatible Fluorescent Dyes |
| BHQ-0 | 430–520 nm | FAM, HEX, TET, JOE, etc. |
| BHQ-1 | 480–580 nm | Suitable for green dyes (e.g., CAL Fluor Orange 560, TET, HEX) |
| BHQ-2 | 559–670 nm | Suitable for yellow to red dyes (e.g., Cy3, Cy5, ROX, Texas Red) |
| BHQ-3 | 620–730 nm | Suitable for far-red dyes (e.g., Cy5.5, Cy7) |
Black Hole Quencher Mechanism
The mechanism of action of BHQs mainly includes FRET and static quenching. Additionally, BHQs can quench fluorescence through a combination of FRET and static quenching, significantly reducing background signals and improving the sensitivity and accuracy of fluorescence detection. This dual mechanism gives BHQs excellent performance in fluorescence detection, making them the quencher of choice in many experiments.
Fluorescence Resonance Energy Transfer
FRET is a physical phenomenon in which energy can be transferred from one fluorescent molecule (donor) to another (acceptor) when they are in close proximity. In the application of BHQs, the fluorescent dye serves as the donor, and BHQ acts as the acceptor. When the fluorescent dye emits light, BHQ absorbs this energy and releases it as heat, thus preventing the dye from fluorescing. This energy transfer process is one of the key mechanisms by which BHQs quench fluorescence.
Static Quenching
In addition to the FRET mechanism, BHQs also quench fluorescence through static quenching. In this process, BHQs form intramolecular dimers with fluorescent dyes, altering the molecular orbital structure of the dyes and preventing them from absorbing and emitting light. These molecular interactions hinder the fluorescence of the dyes, thereby achieving quenching.
Black Hole Quencher Synthesis
The synthesis of BHQ dyes begins with their unique chemical structure design. At their core is a multi-aromatic azo backbone, a structure in which multiple aromatic rings are linked by azo bonds (-N=N-). The introduction of aromatic rings provides a broad π-electron system for the dye, allowing electrons to transition upon light absorption, enabling optical absorption. The azo bonds further enhance the molecule's conjugation effect, allowing electron delocalization over a larger area, thereby modulating the optical properties of the dye. On the basis of the multi-aromatic azo backbone, BHQ dyes are further modified by introducing electron-donating and electron-withdrawing groups on the aromatic rings to adjust their absorption spectra. Electron-donating groups (e.g., amino, hydroxyl) and electron-withdrawing groups (e.g., nitro, cyano) alter the electron distribution of the aromatic rings, thereby affecting the absorption wavelength of the dye. By precisely selecting and adjusting the position and number of these groups, BHQ dyes with absorption across different wavelength ranges can be designed to meet diverse application needs.
Black Hole Quencher Modifications
The diverse modification methods of BHQ dyes provide strong support for their broad application in biomedical research. Through modifications such as amine, carboxylic acid, azide, NHS ester, maleimide, biotin, hydroxyl, and tetrazine, BHQ dyes can be conjugated with various biomolecules or detection systems, enabling efficient regulation and detection of fluorescence signals. These modifications not only enhance the sensitivity and specificity of fluorescence detection but also offer more possibilities for the design and development of novel fluorescent probes.
Black Hole Quencher Amine
BHQ dyes can be modified with active amine groups, allowing them to conjugate with biomolecules containing carboxyl groups (such as proteins, oligonucleotides, etc.) via amide bonds. For example:
- BHQ-1 amine and BHQ-2 amine are common amine-modified products, suitable for post-synthesis labeling.
- Amine-modified BHQ dyes are also used in biosensor preparation to detect target substances by binding to specific biomolecules.
Black Hole Quencher Carboxylic Acid
Carboxylic acid-modified BHQ dyes (such as BHQ-1 acid and BHQ-2 acid) can react with amine-containing molecules through coupling reactions. This modification is commonly used for:
- Reacting with amino groups of proteins, peptides, or oligonucleotides to form stable amide bonds.
- Regulating fluorescence signals by binding with specific biomolecules in fluorescence probe design.
Black Hole Quencher Azide
Azide-modified BHQ dyes (such as BHQ-2-N3 and BHQ-3-N3) introduce azide groups (N₃) that enable participation in click chemistry reactions. This modification has the following characteristics:
- High-efficiency conjugation: Azide groups rapidly form stable triazole rings through reactions with alkynes or cyclooctynes.
- Biocompatibility: Click chemistry reactions proceed under mild conditions, suitable for biological systems.
- Wide application: Commonly used for biomolecule labeling, fluorescence probe construction, and functionalization of nanomaterials.
Black Hole Quencher NHS Ester
NHS ester-modified BHQ dyes (such as BHQ-1 NHS and BHQ-2 NHS) can covalently bind to primary amine-containing biomolecules (such as proteins and peptides). Their characteristics include:
- High reactivity: NHS esters react rapidly with primary amines under mild conditions to form stable amide bonds.
- Wide application: Suitable for protein labeling, fluorescence probe design, and biosensor construction.
Black Hole Quencher Maleimide
Maleimide-modified BHQ dyes (such as BHQ-1 Mal and BHQ-2 Mal) react with sulfhydryl (-SH) groups on biomolecules through maleimide moieties. This modification is suitable for:
- Protein labeling: Reacts with cysteine residues in proteins for specific labeling.
- Fluorescence probe design: Used in constructing probes based on FRET.
Black Hole Quencher Biotin
Biotin-modified BHQ dyes (such as BHQ-1 biotin and BHQ-2 biotin) utilize the strong affinity between biotin and streptavidin. Their characteristics include:
- High affinity: The biotin–streptavidin interaction has an extremely high binding constant, suitable for biomolecule capture and detection.
- Wide application: Commonly used in the separation and purification of proteins and nucleic acids, as well as in fluorescence probe design.
Black Hole Quencher Hydroxyl
Hydroxyl-modified BHQ dyes (such as BHQ-1 OH and BHQ-2 OH) can conjugate with specific biomolecules or materials through hydroxyl groups. This modification can be used for:
- Material functionalization: Conjugation with nanomaterials or polymers to build fluorescent sensors.
- Biomolecule labeling: Regulating fluorescence signals via reactions between hydroxyl groups and biomolecules.
Black Hole Quencher Tetrazine
Tetrazine-modified BHQ dyes (such as BHQ-1 TZ and BHQ-2 TZ) participate in click chemistry reactions through the tetrazine group. This modification has the following characteristics:
- Fast reaction: Tetrazine groups quickly react with trans-cyclooctene (TCO) or dibenzocyclooctyne (DBCO) to form stable covalent bonds.
- Biocompatibility: The reaction conditions are mild and suitable for biological systems.
- Wide application: Commonly used for biomolecule labeling, fluorescence probe construction, and functionalization of nanomaterials.
Applications of Black Hole Quenchers
Black hole quenchers have broad applications in biomedical research, particularly in qPCR techniques and multiplex detection.
qPCR Technology
qPCR is a commonly used gene expression analysis technique that enables quantitative detection of target nucleic acids by real-time monitoring of fluorescence signal changes. In qPCR, the TaqMan probe is one of the most widely used fluorescent probes. The principle of TaqMan probes is based on FRET: the 5' end of the probe is labeled with a fluorescent reporter dye, and the 3' end is labeled with a quencher (such as BHQ). When the probe is intact, the fluorescence emitted by the reporter dye is quenched by BHQ. When the probe hybridizes with the target nucleic acid, the exonuclease activity of Taq polymerase cleaves the probe, separating the reporter dye from BHQ, and the fluorescence signal is restored. By monitoring the fluorescence signal in real-time, the target nucleic acid can be quantitatively detected. As the quencher in TaqMan probes, BHQ offers significant advantages. First, BHQ's non-fluorescent nature results in extremely low background signals, enhancing detection sensitivity. Second, the broad absorption range of BHQ allows compatibility with various fluorescent reporter dyes to meet different experimental needs. Additionally, BHQ has high chemical stability and is resistant to degradation, ensuring experimental reliability.
Multiplex Detection
In biomedical research, multiplex detection is a common experimental method that enhances efficiency and data throughput by simultaneously detecting multiple target molecules. The broad absorption spectrum of BHQ dyes allows compatibility with multiple fluorescent dyes for single-tube multiplex detection. For instance, in multiplex qPCR experiments, BHQ-1, BHQ-2, and BHQ-3 can be used as quenchers, each paired with different wavelength fluorescent reporter dyes, enabling simultaneous detection of multiple genes. This multiplex detection technology is valuable in gene expression analysis, pathogen detection, and disease diagnostics.
Conclusion
With the continuous development of nanotechnology, single-molecule detection, and super-resolution microscopy, fluorescence detection technologies are advancing toward higher sensitivity and resolution. BHQ dyes are expected to play a significant role in these emerging fluorescence detection technologies. For example, in single-molecule fluorescence detection, BHQ's low background interference can significantly enhance sensitivity; in super-resolution microscopy, the broad absorption range of BHQ allows multicolor imaging when used with various fluorescent dyes.
Resources

- Hoechst Dyes: Definition, Structure, Mechanism and Applications
- Mastering the Spectrum: A Comprehensive Guide to Cy3 and Cy5 Dyes
- Fluorescent Probes: Definition, Structure, Types and Application
- Fluorescent Dyes: Definition, Mechanism, Types and Application
- Coumarin Dyes: Definition, Structure, Benefits, Synthesis and Uses
- Unlocking the Power of Fluorescence Imaging: A Comprehensive Guide
- Cell Imaging: Definitions, Systems, Protocols, Dyes, and Applications
- Lipid Staining: Definition, Principles, Methods, Dyes, and Uses
- Flow Cytometry: Definition, Principles, Protocols, Dyes, and Uses
- Nucleic Acid Staining: Definition, Principles, Dyes, Procedures, and Uses
Online Inquiry

