How to Select the Best Fluorophore for Reliable Antibody Labeling Results?
Antibody labeling is an indispensable tool in modern life science research and diagnostic technologies. By conjugating antibodies with fluorophores, it enables highly sensitive and specific detection and visualization of target molecules. Choosing the right fluorophore not only affects signal intensity and image quality but also directly impacts the reliability of experiments and the reproducibility of data. In complex applications such as multicolor imaging, flow cytometry, or in vivo imaging, factors like excitation/emission wavelengths, photostability, brightness, and chemical compatibility of different fluorophores can significantly influence experimental outcomes. Therefore, scientifically evaluating fluorophore properties and matching them to experimental requirements is crucial for achieving high-quality antibody labeling results.
What are Fluorescently Labeled Antibodies?
Fluorescently labeled antibodies are products in which a fluorophore, either covalently or non-covalently, is attached to an antibody to enable optical detection of antigen–antibody interactions. Fluorescent labeling allows direct observation of target molecules in various biological assays, including flow cytometry, immunofluorescence microscopy, fluorescent Western blotting, and ELISA. Fluorescent antibodies can be classified into direct labeling, where the fluorophore is directly conjugated to the primary antibody (direct fluorescent antibody labeling), and indirect labeling, where an unlabeled primary antibody captures the antigen and a fluorescently labeled secondary antibody amplifies the signal (using fluorescent labeled secondary antibody). Different labeling strategies can be flexibly selected based on experimental needs.
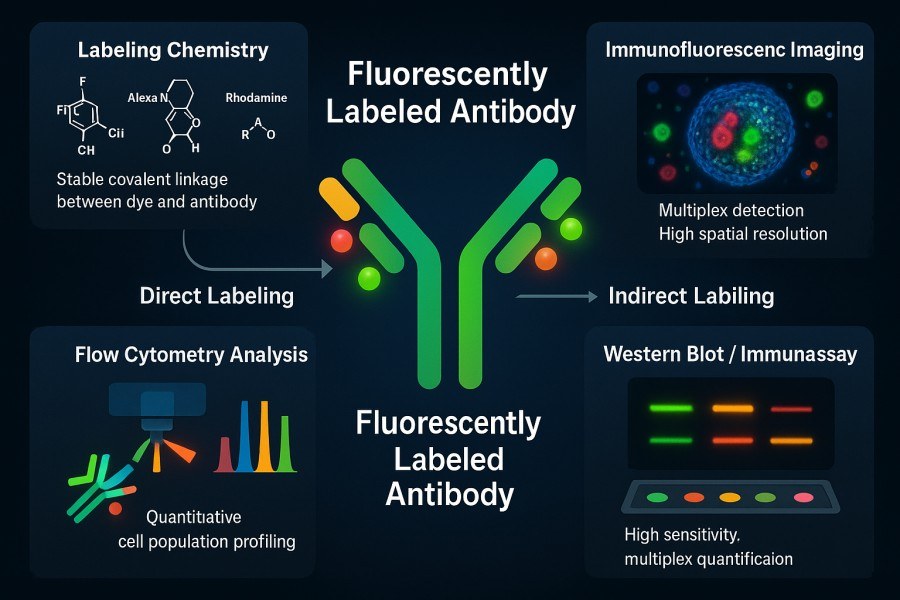 Fig. 1. Fluorescent labeling of antibodies (BOC Sciences Authorized).
Fig. 1. Fluorescent labeling of antibodies (BOC Sciences Authorized).
The Mechanism of Fluorescence in Biological Detection
The principle of fluorescence detection is based on electronic transitions in fluorophore molecules. Fluorophores have conjugated double-bond systems that, upon illumination with light of specific wavelengths, are excited to a higher energy state and emit longer-wavelength photons when returning to the ground state. This excitation-emission process can be separated using filters and detection devices, allowing highly sensitive detection of specifically labeled targets in biological samples. Fluorescent labeling techniques do not require radioactive tracers and are relatively simple to operate, making them widely used in life sciences and diagnostics.
Importance of Choosing the Right Fluorophore for Accurate Results
Accurate and reliable results require selecting the appropriate fluorophore. Different fluorophores vary in excitation/emission spectra, quantum yield, and stability. Improper selection can lead to signal overlap, increased background, or signal loss. For instance, in multiplex experiments, significant overlap between emission spectra of multiple fluorophores can cause signal "bleeding" from one channel to another, producing erroneous readings (known as fluorescence crosstalk), and even correction may reduce accuracy. Therefore, from the experimental design stage, it is essential to match fluorophore optical properties with the excitation source (laser wavelength), detection channels (filter bandwidths), and intended application to ensure that each fluorescent antibody is uniquely and efficiently excited and detected.
Key Factors to Consider When Selecting a Fluorophore
Fluorophore selection is a critical factor affecting the success of antibody labeling experiments. Fluorophores differ significantly in excitation and emission spectra, brightness, photostability, and environmental sensitivity, which directly determine signal intensity, signal-to-noise ratio, and experimental reproducibility. The following four key factors should be prioritized when designing fluorescent antibody experiments:
- Excitation and Emission Spectra Compatibility: The selected fluorophore's excitation and emission peaks must match the experimental equipment, such as laser wavelengths and filter sets. For example, if using a 488 nm laser, the fluorophore should have an excitation peak near 488 nm (e.g., FITC, Alexa Fluor 488) and an emission peak within the detection channel range. In multicolor experiments, peaks should be well separated to minimize spectral overlap between fluorophores.
- Brightness and Quantum Yield: Brightness, defined as the product of molar extinction coefficient and quantum yield, reflects overall emission efficiency. Higher brightness results in more photons emitted under the same excitation, improving signal-to-noise ratio and detection of low-abundance targets. Fluorophores with the highest brightness in the available excitation range are generally preferred, but brightness must be balanced with other properties, as high-brightness dyes may require specific buffer conditions.
- Photostability for Long-Term Imaging: Photostability indicates a fluorophore's resistance to photobleaching under light exposure. In long-term or high-intensity experiments, such as confocal microscopy or multicolor flow cytometry, photobleaching causes signal decay over time. Ideal fluorophores should have a long bleaching half-life (bleaching t½). For live-cell time-lapse imaging, dyes with high photostability are preferred to maintain consistent signal throughout imaging.
- pH Sensitivity and Environmental Stability: Fluorescence performance may change with pH, temperature, and environmental polarity. Some fluorophores, like FITC, lose fluorescence under acidic conditions, while others are sensitive to polarity changes. Knowledge of the fluorophore's pKa is important (e.g., FITC pKa ≈ 6.4, nearly non-fluorescent at low pH). Additionally, some dyes are prone to hydrolysis or oxidation and must be stored protected from light at low temperature. Selection should consider the expected physiological pH and sample preparation conditions to ensure stable fluorescence.
Comparison of Commonly Used Fluorophores
A wide variety of fluorophores are available commercially, each with distinct excitation/emission wavelengths, brightness, photostability, chemical reactivity, and environmental adaptability. Scientific comparison and proper selection of fluorophores are essential for achieving high-sensitivity detection and multiplex labeling. The following is a systematic comparison of commonly used fluorophores, including FITC, TRITC, Rhodamine series, Alexa Fluor, DyLight series, Cyanine series, and near-infrared (NIR) dyes, to guide researchers and companies in choosing the most suitable labeling strategy.
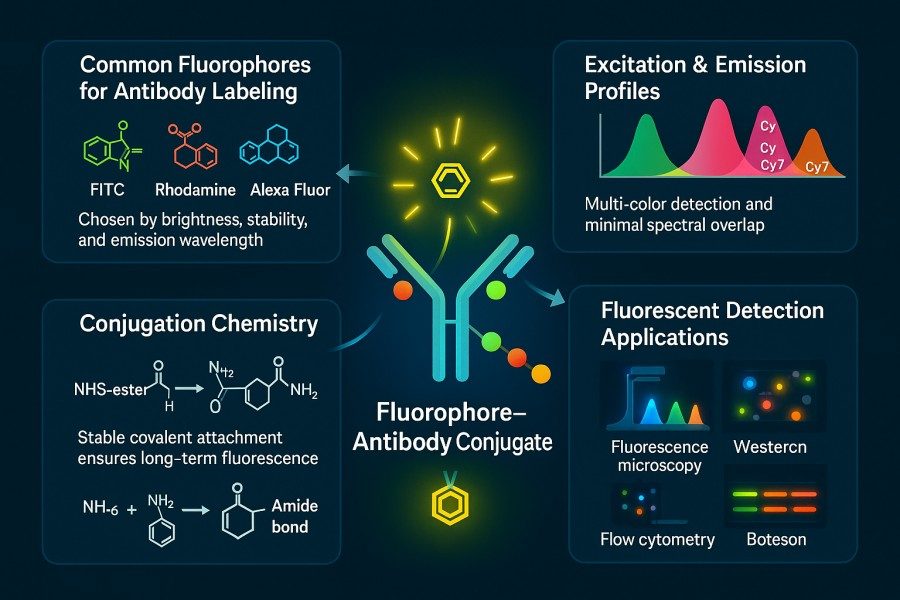 Fig. 2. Fluorophores for antibody labeling (BOC Sciences Authorized).
Fig. 2. Fluorophores for antibody labeling (BOC Sciences Authorized).
FITC, TRITC, and Rhodamine Series
FITC (fluorescein isothiocyanate) is a classic green dye with high molar absorptivity and quantum yield but has limitations: broad emission spectrum, rapid photobleaching under strong light, acid sensitivity, and partial quenching upon protein conjugation. TRITC (tetramethylrhodamine isothiocyanate) is orange-red, with absorption ~550 nm and emission ~570 nm, more resistant to acidic conditions than FITC but still photobleaches relatively quickly. The Rhodamine family (e.g., Texas Red) emits at longer wavelengths with lower background autofluorescence, though brightness is lower than newer dyes. These traditional dyes suit general applications but require consideration of stability and spectral properties.
Alexa Fluor and DyLight Dyes
Alexa Fluor dyes are high-performance fluorophores covering blue to infrared wavelengths, offering high brightness and excellent photostability, commonly used in demanding multicolor experiments. DyLight dyes also provide a wide color range, with higher stability in some wavelengths, particularly in the long-wavelength region. For example, Alexa Fluor 647 and DyLight 650 perform well in the far-red region with superior photostability compared to traditional rhodamines. Overall, Alexa and DyLight series are widely preferred for modern fluorescent labeling.
Cyanine Dyes (Cy3, Cy5, Cy7)
Cyanine dyes are known for high molar extinction coefficients and narrow spectra, suitable for high-sensitivity detection. Cy3 (~550 nm emission) and Cy5 (~670 nm emission) absorb strongly from UV to near-infrared and are commonly used in FRET and gene chip analysis. Cy7 (~780 nm emission) is ideal for in vivo small animal imaging. Cyanine dyes are highly fluorescent and chemically tunable to cover almost the entire visible to NIR range but are sensitive to light and chemical environment, requiring stabilizers.
Near-Infrared (NIR) Dyes for Deep Tissue Imaging
For deep tissue or in vivo imaging, NIR dyes (~700–900 nm) offer strong tissue penetration and minimal background autofluorescence. Common NIR fluorophores include indocyanine green (ICG) and specialized dyes like IRDye and Biotium NIR CF®. These dyes typically feature excellent photostability and water solubility, making them suitable for live-cell labeling and small animal imaging. NIR dye use requires specialized detection equipment and filters, so they are selected only when the imaging system supports the corresponding wavelengths.
Common Fluorescent Fluorophores for Antibody Labeling
| Catalog | Name | CAS | Fluorescence Color | Ex (nm) | Em (nm) | Main Applications | Inquiry |
|---|---|---|---|---|---|---|---|
| F01-0045 | BODIPY 505/515 | 21658-70-8 | Green | 505 | 515 | Visualizing lipid droplets in microalgae; fluorescence microscopy applications | Bulk Inquiry |
| F01-0044 | BODIPY-Cholesterol | 878557-19-8 | Green | 505 | 515 | Cholesterol trafficking studies; lipid membrane labeling | Bulk Inquiry |
| R02-0024 | Cyanine7 alkyne | 1998119-13-3 | Near-Infrared | 743 | 767 | In vivo imaging; click chemistry conjugation | Bulk Inquiry |
| F02-0006 | Cyanine3.5 carboxylic acid | 1802928-88-6 | Green | 520 | 540 | Nucleic acid and protein labeling; qPCR; RNA/DNA isolation | Bulk Inquiry |
| F03-0009 | Sulfo-Cyanine5.5 amine | 2183440-45-9 | Far-Red | 675 | 695 | In vivo imaging; antibody labeling; flow cytometry | Bulk Inquiry |
| F02-0013 | Cyanine5.5 maleimide | 1594414-90-0 | Far-Red | 675 | 695 | Thiol-reactive conjugation; protein labeling | Bulk Inquiry |
| R01-0042 | AF594 activated ester, 5-isomer | 1638544-48-5 | Red | 590 | 617 | Antibody labeling; fluorescence microscopy; flow cytometry | Bulk Inquiry |
| R01-0471 | AF647 NHS ester | 407627-60-5 | Far-Red | 650 | 665 | Antibody labeling; in vivo imaging; multiplex assays | Bulk Inquiry |
| R01-0039 | AF430 NHS ester | 467233-94-9 | Blue | 430 | 440 | Antibody labeling; flow cytometry; fluorescence microscopy | Bulk Inquiry |
| R01-0451 | AF 488 TFP ester | 2133404-55-2 | Green | 495 | 519 | Antibody labeling; flow cytometry; microscopy | Bulk Inquiry |
| F05-0031 | 6-Carboxy-X-rhodamine | 194785-18-7 | Orange-Red | 580 | 605 | Protein labeling; fluorescence microscopy; flow cytometry | Bulk Inquiry |
| A16-0014 | Sulforhodamine 101 | 60311-02-6 | Red | 583 | 604 | Cell viability assays; fluorescence microscopy; flow cytometry | Bulk Inquiry |
| A16-0093 | Rhodamine 6G | 989-38-8 | Orange-Red | 530 | 552 | Fluorescence microscopy; flow cytometry; laser dye | Bulk Inquiry |
| A01-0005 | Rhodamine B | 81-88-9 | Orange-Red | 540 | 625 | Fluorescence microscopy; dye laser; protein labeling | Bulk Inquiry |
| A17-0016 | Rhodamine 6G Perchlorate | 13161-28-9 | Orange-Red | 530 | 552 | Fluorescence microscopy; flow cytometry; laser dye | Bulk Inquiry |
| A18-0008 | Rhodamine 110 chloride | 13558-31-1 | Green | 502 | 518 | Fluorescence microscopy; flow cytometry; ion channel studies | Bulk Inquiry |
| F04-0012 | FITC isomer I | 3326-32-7 | Green | 495 | 519 | Antibody labeling; flow cytometry; fluorescence microscopy | Bulk Inquiry |
| F04-0055 | Dexamethasone Fluorescein | 216854-76-1 | Green | 495 | 519 | Steroid receptor studies; fluorescence microscopy | Bulk Inquiry |
| R10-0005 | 6-Fluorescein phosphoramidite | 204697-37-0 | Green | 494 | 521 | Nucleic acid labeling; oligonucleotide synthesis | Bulk Inquiry |
| A16-0033 | 6-Carboxyfluorescein | 3301-79-9 | Green | 494 | 521 | Nucleic acid labeling; fluorescence microscopy; flow cytometry | Bulk Inquiry |
| A15-0005 | 5(6)-Carboxyfluorescein | 72088-94-9 | Green | 494 | 521 | Nucleic acid labeling; fluorescence microscopy; flow cytometry | Bulk Inquiry |
Looking for Antibody Labeling Dyes?
We provide flexible conjugation options with various fluorophores, including water-soluble and photostable dyes, to meet your experimental requirements.
Matching Fluorophores to Experimental Applications
Different experimental platforms have distinct requirements for fluorescently labeled antibodies, making it critical to match fluorophores to specific applications. Whether for flow cytometry, immunofluorescence confocal microscopy, Western blot, ELISA, or in vivo imaging, each application has particular demands regarding brightness, photostability, spectral width, and environmental adaptability. Understanding these requirements and selecting fluorophores accordingly can prevent signal interference and low sensitivity, while improving experimental reproducibility and data accuracy.
Flow Cytometry and Multiplex Detection
Flow cytometry often requires simultaneous detection of multiple markers, and multicolor flow cytometry imposes stringent requirements on fluorophore spectral compatibility. When designing a multicolor panel, fluorophores should be "matched" to each labeled antibody, aligning their spectral properties with the instrument's lasers and filters while avoiding dyes with high spectral overlap. For instance, different lasers can excite different fluorophores, and signals are collected in separate detector channels. Tandem dyes (e.g., PE-Cy7) are frequently used to extend the number of available channels and increase excitation-emission separation. In panel design, low-abundance markers should be paired with the brightest dyes, while highly expressed targets may use moderately bright dyes with better spectral placement to optimize overall sensitivity. It is recommended to use fluorophore spectral viewers or the instrument's spillover spread matrix (SSM) analysis before experiments to predict and correct channel crosstalk.
Immunofluorescence and Confocal Microscopy
Immunofluorescence and confocal microscopy require high photostability and low background autofluorescence. In addition to matching microscope filters, photobleaching resistance is critical, as confocal imaging involves high-intensity excitation and often prolonged acquisition times. Common fluorescent proteins (e.g., GFP, mCherry) and small-molecule dyes (e.g., Alexa Fluor, DyLight series) maintain long fluorescence lifetimes when used with antifade mounting media. For experiments requiring tissue penetration or with high sample autofluorescence, near-infrared dyes may be considered to improve signal-to-noise ratios.
Western Blotting and ELISA Applications
Traditional Western blotting and ELISA often use enzyme-based detection, but fluorescence provides a non-radioactive alternative with a wide dynamic range. Fluorescent Western blots commonly employ near-infrared secondary antibodies (e.g., LI-COR IRDye 680/800 series) that emit around 800 nm, offering minimal background and high sensitivity. Fluorescent probes in ELISA (e.g., fluorescein substrates or labeled secondary antibodies) also require spectral compatibility; for example, chemifluorescent ELISA substrates are detected at their specific emission wavelengths. Overall, dyes should have absorption near the instrument's excitation source, well-defined emission peaks, and low photobleaching to ensure accurate quantitative results.
In Vivo Imaging Considerations
For in vivo or live-animal imaging, longer-wavelength near-infrared (NIR, ~700–900 nm) fluorophores are preferred. NIR light penetrates tissues more effectively, and endogenous autofluorescence is minimal, enhancing imaging sensitivity. Common NIR fluorophores include indocyanine green (ICG) and various NIR cyanine dyes. NIR dyes developed by companies such as Biotium exhibit high brightness and photostability, making them ideal for deep-tissue small-animal imaging. For experiments requiring dynamic monitoring of antibodies or labeled cells in vivo, these long-wavelength dyes should be prioritized.
Avoiding Common Pitfalls in Fluorophore Selection
Despite widespread use, fluorescent antibody labeling has potential pitfalls, including spectral overlap, chemical incompatibility, over-labeling, or mismatch between dye and detection system. Familiarity with these issues is essential to ensure smooth experiments and high-quality results.
- Overlapping Spectra and Signal Crosstalk: A common issue in multicolor experiments is spectral overlap. If not carefully managed, a dye may emit outside its intended detection channel, causing signal "bleed." This leads to measurement errors, and even compensation algorithms may amplify inaccuracies. To avoid this, choose fluorophore combinations with well-separated spectra and perform one-time crosstalk correction in the same experimental setup. Tandem dyes can reduce overlap in some channels but require attention to their stability and usage conditions (e.g., avoid freezing).
- Incompatible Labeling Chemistries: Different fluorophores require specific reactive groups to conjugate with antibodies. Common chemistries include NHS esters (reacting with protein amines under basic conditions) and maleimides (reacting with thiols). Using a dye incompatible with available antibody functional groups results in low conjugation efficiency. For example, NHS ester dyes react with free amino groups (–NH₂) on antibodies to form stable amide bonds. If lysines are removed during antibody production or thiols are insufficient, the wrong reactive dye will fail to conjugate. Buffer components containing amines or thiol inhibitors can also compete, causing conjugation failure. It is essential to remove interfering substances and select activation methods based on antibody composition and functional groups.
- Over-Labeling and Loss of Antibody Activity: Excessive dye conjugation can cause self-quenching or steric hindrance, reducing antibody binding activity. Typically, each IgG can carry 4–5 dye molecules without significant functional loss. Over-labeling leads to energy transfer between adjacent dyes and quenching, and large dye molecules may block antigen-binding sites, reducing affinity. Optimizing dye-to-antibody molar ratios and performing small-scale tests to determine ideal labeling density, monitored via absorbance or fluorescence, helps avoid these issues.
- Mismatch Between Dye and Detection System: If the chosen fluorophore cannot be efficiently excited by the instrument's light source or its emission does not fall within detector channels, no signal can be detected. Always verify excitation wavelengths and filter configurations of the instrument to ensure proper excitation and detection of each dye. Cross-validation of instrument parameters and fluorophore spectra before experiments prevents wasted resources due to spectral mismatch.
Practical Considerations for Fluorophore–Antibody Conjugation
Successful fluorescent antibody labeling depends not only on the fluorophore but also on optimized conjugation strategies and operational conditions to maintain antibody quality, stability, and functionality. Appropriate labeling ratios, compatible buffers, efficient chemical reactions, and rigorous purification and characterization are key to experimental success.
Labeling Ratios and Optimization Strategies
The molar ratio of dye to antibody and reaction time directly affect labeling density (number of dyes per antibody). Ideally, each IgG carries ~4–5 fluorophores to balance signal and activity. Accurately measure antibody concentration before reaction, calculate dye amounts, and optimize in batches. Post-labeling, measure absorbance and fluorescence to calculate actual labeling density, and purify using chromatography or centrifugal columns to remove unbound dye. Ensuring proper labeling maximizes fluorescence without compromising antibody affinity.
Buffer Conditions and Reactive Groups (NHS, Maleimide)
The buffer must support the chosen chemical reaction. NHS ester dyes react best in bicarbonate or PBS buffers at ~pH 8.3, while maleimide dyes react with reduced thiols under neutral to slightly reducing conditions (pH ~6.5–7.5). Buffers should avoid substances that compete with reactive groups (e.g., Tris, amines, thiols, blocking agents). NHS ester conjugation involves activation of carboxyl groups under basic conditions to form stable amide bonds with antibody amino groups. Maintain appropriate pH, temperature, and mixing to enhance reaction efficiency and minimize side reactions.
Purification and Quality Validation of Labeled Antibodies
After labeling, remove free dye (via dialysis, ultrafiltration, or gel filtration) to reduce background. Validate labeled antibodies using UV–Vis spectrophotometry to calculate dye-to-antibody ratios and SDS-PAGE with fluorescence imaging to confirm molecular weight and fluorescence integrity. Functional assays (e.g., binding experiments) can further confirm antibody activity.
Storage and Stability of Fluorescent Conjugates
Fluorescent antibodies should be stored protected from light at low temperatures (typically -20°C or 4°C with preservatives). Some dyes are hygroscopic, prone to hydrolysis, or photobleach easily; storage at -80°C or on dry ice can extend stability. Thaw frozen conjugates slowly to room temperature to avoid condensation affecting activity. Proper storage ensures stable fluorescence performance during use.
How BOC Sciences Supports Custom Fluorescent Antibody Development?
Leveraging extensive expertise in biochemistry and molecular labeling, BOC Sciences provides end-to-end custom services, from fluorophore synthesis and structural modification to optimized antibody conjugation and labeled product characterization. This comprehensive approach ensures researchers and companies obtain fluorescently labeled antibodies with high brightness, excellent stability, and preserved activity, enhancing experimental reproducibility and data reliability.
Fluorescein-Based Antibody Labeling
- Supports labeling with various fluorescein derivatives, such as FITC and its water-soluble modified forms.
- Offers both direct and indirect labeling strategies to maintain antibody activity and signal strength.
- Custom conjugation chemistry optimization is available to accommodate different antibody types.
- Enables multicolor labeling experiments while minimizing background interference.
Rhodamine-Based Antibody Labeling
- Provides conjugation services for TRITC, Rhodamine B, Rhodamine 6G, and other derivatives.
- Enables efficient covalent labeling of proteins, antibodies, and nanomaterials.
- Supports fluorescence modification and functionalization for versatile applications.
- Offers optimized conjugation conditions to ensure signal stability and strong fluorescence.
Cyanine Dye Antibody Labeling
- Provides conjugation services for common cyanine dyes such as Cy3, Cy5, and Cy7.
- Supports both direct and indirect labeling strategies to meet diverse experimental needs.
- Custom dye modifications are available to enhance water solubility and photostability.
- Offers multi-channel labeling solutions for multiplex fluorescent imaging.
Alexa Fluor Antibody Labeling
- Supports antibody labeling with the Alexa Fluor series, including Alexa Fluor 488, 555, and 647.
- Enables efficient conjugation with small molecules or proteins.
- Provides optimized conjugation strategies to ensure uniform labeling and high signal-to-noise ratio.
- Supports both direct and indirect fluorescent antibody applications, suitable for research and diagnostic experiments.
Do You Need A Consultation?
BOC Sciences integrates cutting-edge fluorescence technologies to accelerate your research, driving next-generation solutions for drug discovery and diagnostics.
Transform Your Studies with Cutting-Edge Fluorescent Products
| Cat. No. | Name | CAS No. | Inquiry |
|---|---|---|---|
| F01-0012 | 3-Bodipy-propanoic acid | 165599-63-3 | Bulk Inquiry |
| F01-0064 | meso-CH2Br-BODIPY | 216434-81-0 | Bulk Inquiry |
| A16-0033 | 6-Carboxyfluorescein | 3301-79-9 | Bulk Inquiry |
| A16-0201 | DAPI dihydrochloride | 28718-90-3 | Bulk Inquiry |
| A15-0005 | 5(6)-Carboxyfluorescein | 72088-94-9 | Bulk Inquiry |
| F04-0033 | 5-Aminofluorescein | 3326-34-9 | Bulk Inquiry |
| F04-0034 | 5-Carboxyfluorescein diacetate | 79955-27-4 | Bulk Inquiry |
| F05-0031 | 6-Carboxy-X-rhodamine | 194785-18-7 | Bulk Inquiry |
| F01-0254 | BODIPY 493/503 carboxylic acid | 216961-95-4 | Bulk Inquiry |
| F03-0022 | Sulfo-Cyanine 5 Carboxylic Acid (ethyl) | 146368-11-8 | Bulk Inquiry |
High-Performance Fluorescent Tools for Your Research
- TAMRA Dyes Red-emitting dyes for antibody and protein labeling.
- ATTO Dyes High-performance dyes for labeling and imaging experiments.
- Coumarin Blue-emitting dyes for chemical sensing and fluorescence studies.
- Fluorescent Protein Used for live-cell imaging and real-time biosensing.
More About Antibody labeling
Online Inquiry

