Fluorescent-Labeled Antibodies for Flow Cytometry: Maximize Sensitivity and Accuracy
Flow cytometry is one of the most critical quantitative analysis techniques in modern immunology, cell biology, and biomedical research, while fluorescent-labeled antibodies are the core tools enabling high-sensitivity, multiparameter detection. With the advancement of multicolor flow cytometry, spectral cytometry, and high-dimensional cell analysis, researchers' requirements for antibody quality, labeling methods, and fluorophore selection have been steadily increasing.
What Are Fluorescent-Labeled Antibodies?
Fluorescent-labeled antibodies are biological probes constructed by covalently conjugating a fluorophore to an antibody molecule, allowing precise recognition of specific target antigens at the cellular level. This conjugation preserves the antibody's natural binding ability while providing a fluorescent signal detectable by a flow cytometer, playing a key role in quantitative and multiparameter analysis.
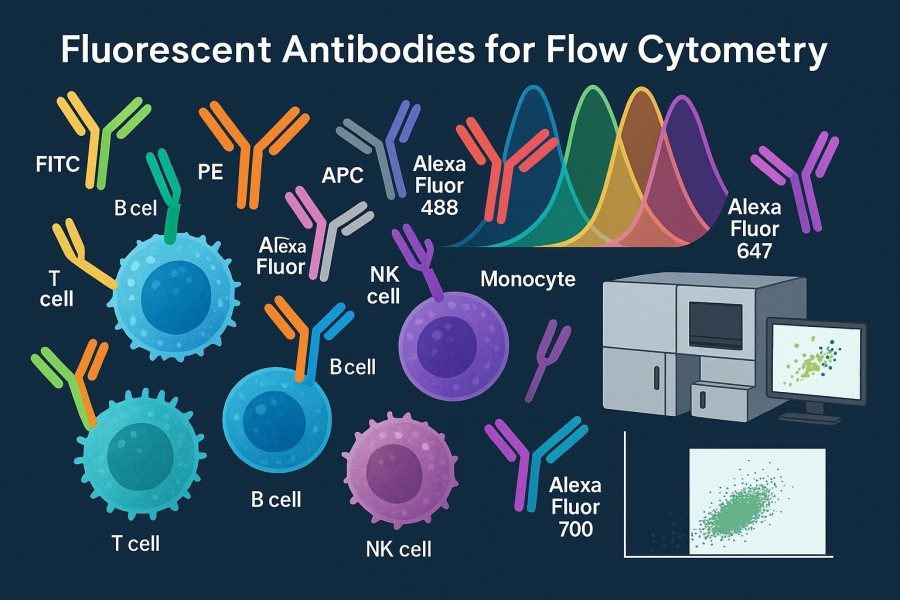 Fig. 1. Fluorescent antibodies for flow cytometry (BOC Sciences Authorized).
Fig. 1. Fluorescent antibodies for flow cytometry (BOC Sciences Authorized).
In flow cytometry, each fluorescent-labeled antibody represents a detection parameter. Researchers can use these antibodies to perform high-throughput detection of cell surface proteins, intracellular signaling molecules, transcription factors, cytokines, and more. Fluorescent-labeled antibodies not only help identify immune cell subsets but also reveal cellular functional states, activation levels, differentiation pathways, and biological heterogeneity.
With the maturation of multicolor and spectral flow cytometry, the importance of fluorescent-labeled antibodies has further increased. Modern flow cytometry experiments often use combinations of 10, 20, or even 30 fluorescent-labeled antibodies, imposing stricter requirements on antibody quality, fluorophore stability, spectral properties, and conjugation consistency. High-quality fluorescent antibodies provide high signal-to-noise data and significantly improve experimental reproducibility and analytical depth.
How Flow Cytometry Detects Fluorescent Signals?
The core of flow cytometry is rapid optical analysis of individual cells. Its fluorescence detection mechanism is based on cells forming a single-cell stream within sheath fluid and sequentially passing through multiple laser beams. When the laser excites fluorophores on or within the cell, they emit light at specific wavelengths. The flow cytometer collects this emitted light through an optical system and transmits it to photomultiplier tubes (PMTs), avalanche photodiodes (APDs), or spectral detectors for signal conversion and amplification. Fluorescence detection efficiency and accuracy are influenced by several optical factors:
- Laser-fluorophore spectral matching determines signal strength: Each fluorophore has optimal excitation and emission peaks. Stronger, lower-background signals are obtained when the instrument's laser wavelength closely matches the fluorophore's excitation spectrum. For example, PE is optimal for 488 nm or 561 nm lasers, while APC works best with 633–640 nm lasers.
- Optical filter combinations affect signal separation: In multicolor experiments, emission spectra of fluorophores often overlap. Appropriate bandpass filters are essential for distinguishing adjacent signals, directly impacting spectral spillover and compensation accuracy.
- Detector sensitivity determines weak signal detectability: High-sensitivity PMTs or digital APDs can capture very low fluorescence emissions, especially important for low-abundance antigens or dim fluorophores. Detector performance varies significantly across instrument platforms, a crucial factor in panel design.
In spectral flow cytometry, fluorescence signals are no longer captured by a single filter but are dispersed into continuous spectra by prisms and diffraction gratings, then computationally reconstructed to determine each fluorophore's contribution. This allows researchers to more accurately distinguish multiple fluorophores, greatly enhancing multiparameter analysis capability.
The Role of Antibody–Fluorophore Conjugation in Cell Analysis
The conjugation of antibodies to fluorophores is the critical step determining fluorescent-labeled antibody quality, directly affecting signal intensity, background levels, antibody specificity, and experimental reproducibility. High-quality antibody-fluorophore conjugation requires high-purity materials and strict control of reaction conditions, molar ratios, and subsequent purification. Key attributes of high-quality conjugates include:
- Appropriate degree of labeling (DOL) to preserve antibody structure: DOL refers to the number of fluorophores attached per antibody molecule. Low DOL results in weak signals, while excessive DOL can disrupt antibody conformation, hinder antigen binding, or cause self-quenching. Accurate DOL control ensures antibody activity and signal quality.
- Avoidance of self-quenching: High fluorophore density may cause energy interactions between molecules, reducing signal strength, especially with FITC or Cy5. Optimizing conjugation conditions and selecting suitable spacers reduces this risk.
- Strong and specific signals: High-quality conjugation ensures antibodies maintain full target recognition post-labeling, whether for surface or intracellular antigens. Fluorophores must also have sufficient brightness and photostability for extended acquisition and repeated laser excitation.
- Improved sensitivity and reproducibility: Stable conjugation efficiency, uniform molecular structure, rigorous purification, and QC verification ensure consistent data across batches and experimental systems, minimizing errors caused by antibody quality variability.
As experiments become more complex, particularly in panels with over 10 colors, the importance of high-quality antibody-fluorophore conjugation increases. Quality antibodies reduce compensation burden, minimize background noise, and enhance population discrimination, providing a solid foundation for immune monitoring, drug analysis, and high-dimensional cell profiling.
How Do Fluorescent Antibodies Work in Flow Cytometry?
Fluorescent antibodies function based on highly specific antigen–antibody recognition and quantitative detection of fluorescence signals. By conjugating fluorophores to antibodies, researchers can capture protein expression, signaling pathway activation, and cell subset distribution at the single-cell level in real time. This combination of chemical labeling and optical detection allows flow cytometry to achieve high-throughput, multiparameter, quantitative, and high-sensitivity analysis.
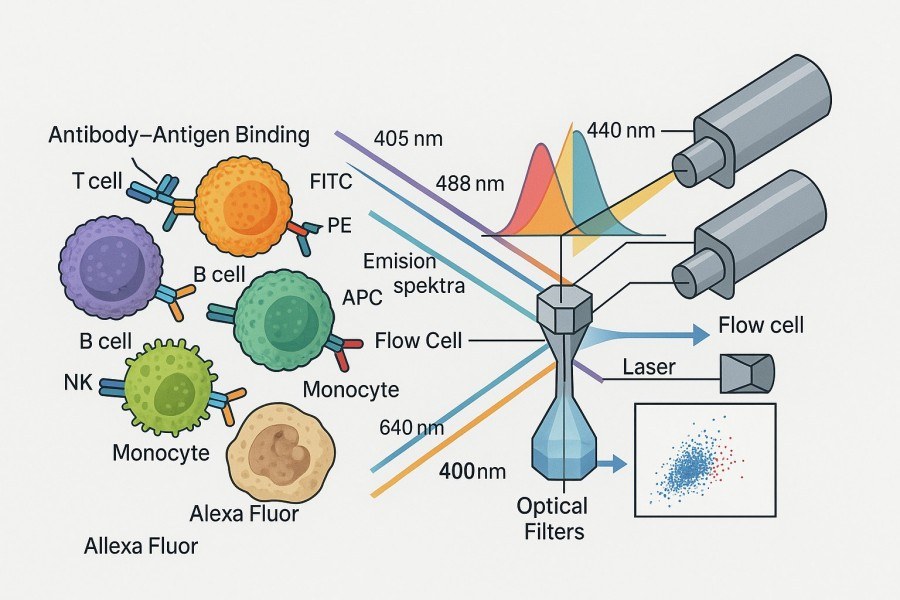 Fig. 2. Principles of flow cytometric detection using fluorescent antibodies (BOC Sciences Authorized).
Fig. 2. Principles of flow cytometric detection using fluorescent antibodies (BOC Sciences Authorized).
When target cells flow as single-cell suspensions through the cytometer, protein information represented by fluorescent antibodies is excited by lasers of specific wavelengths, producing fluorescence emission signals that are converted into quantifiable data by optical and electronic detection modules. The process operates at millisecond response times, enabling detection of tens of thousands to millions of cells within a very short period.
Antigen–Antibody Binding and Fluorescence Detection
Fluorescent antibody signals originate from highly specific antigen–antibody binding and the energy release of fluorophores upon light excitation. Once antibodies bind their target proteins, the corresponding fluorophores localize to specific positions on the cell surface or intracellular compartments. As each cell passes through the laser beam, the fluorophores absorb photons, transition to higher energy states, and then return to the ground state while emitting light at specific wavelengths.
The flow cytometer collects these emitted photons in real time through multiple photodetectors, enabling:
- Quantitative protein expression: Emission intensity is proportional to antigen density, allowing differentiation from low to high expression.
- Distinguishing cells with different biological states: For example, T cell activation markers (CD69, CD25), differentiation status (CD45RA/RO), or exhaustion markers.
- Identifying rare populations: Such as Tregs, memory B cells, or events as rare as one in 100,000.
For low-abundance antigens, high-brightness fluorophores (PE, APC) are essential to enhance signal-to-noise ratio and detection sensitivity. For high-abundance targets, moderate brightness fluorophores prevent signal saturation. Fluorescence signals also depend on DOL, fluorophore photostability, and detector sensitivity. Selecting high-quality fluorescent antibodies is therefore key to reliable data.
Multiparameter Analysis and Spectral Considerations
With advances in modern flow cytometry, multiparameter analysis has expanded from 4–8 colors to 18–30 colors, and spectral flow cytometry can exceed 40 colors, requiring careful consideration of fluorophore spectral properties. Key challenges in multiparameter detection include spectral interactions among fluorophores. Factors to consider include:
- Spectral separation: Fluorophores should be chosen for maximal distinction in excitation/emission spectra to reduce signal overlap. For example, FITC and PE spectra are close and prone to spillover; APC and Alexa Fluor 647 overlap, requiring strict compensation. Proper fluorophore configuration is the first step in optimizing multicolor panels.
- Spillover and compensation: In multicolor experiments, some fluorescence inevitably bleeds into other detection channels. Compensation matrices correct this, relying on single-stained controls, and directly affecting gating analysis accuracy.
- Spectral purity in spectral flow cytometry: Spectral flow captures complete continuous spectra and uses algorithms for unmixing. This allows more fluorophores to be resolved but requires unique fluorophore spectra, high antibody labeling purity, and rigorous instrument calibration to ensure accurate analysis.
Choosing the Right Fluorophores for Flow Cytometry
Fluorophore selection determines experiment sensitivity, resolution, and multicolor panel scalability. Proper fluorophore combinations enhance brightness, reduce compensation complexity, and improve data stability and reproducibility. For researchers performing immunophenotyping, cell profiling, or high-dimensional flow experiments, optimizing fluorophore choice is crucial for reliable results.
Factors to Consider: Brightness, Photostability, and Spectral Compatibility
When selecting fluorophores, consider:
- Brightness: High brightness is critical for detecting low-expression antigens, especially in multicolor panels targeting rare cells or weak signals.
- Photostability: Fluorophores resistant to photobleaching maintain consistent signals during prolonged exposure or repeated laser excitation.
- Spectral compatibility: Avoid overlapping excitation/emission spectra to minimize channel spillover, reduce compensation requirements, and improve data quality.
- Instrument laser compatibility: Different flow cytometers use varying laser wavelengths (e.g., 405 nm, 488 nm, 561 nm, 633 nm), so fluorophores must match available laser lines for optimal excitation.
In multicolor panel design, assign high-brightness fluorophores to low-expression antigens and moderate-brightness fluorophores to high-expression targets for balanced signal distribution.
Commonly Used Fluorophores (FITC, PE, APC, PerCP, Alexa Fluor Series)
Different fluorophores have unique optical properties, brightness, and excitation/emission ranges, playing critical roles in multicolor panel design. Understanding these characteristics helps researchers select appropriate fluorophores to maximize sensitivity and data reliability.
- FITC (Fluorescein Isothiocyanate): A classic green fluorophore with well-defined excitation/emission spectra and stable performance. Its moderate brightness is suitable for high-expression antigens. FITC is slightly less photostable than PE or Alexa Fluor dyes but remains widely used for its cost-effectiveness, ease of compensation, and broad applicability.
- PE (Phycoerythrin): One of the brightest fluorophores in flow cytometry, enabling detection of low-expression or rare antigens. High quantum yield and strong emission peaks make it ideal for key targets in large panels and as a base for tandem dyes (e.g., PE-Cy5, PE-Cy7) to expand multicolor capability.
- APC (Allophycocyanin): Emits in the far-red region with low background and high brightness, suitable for high-sensitivity detection in complex panels. APC's spectral properties facilitate compensation and excel in deep immunophenotyping, multiparameter analysis, and low-frequency cell detection. APC can also form tandem dyes (e.g., APC-Cy7).
- PerCP: Efficiently excited by 488 nm lasers and commonly used in classical immunophenotyping (e.g., CD45, CD3). Its spectra complement FITC and PE, providing clear signal separation in four-color or basic panels. Although less bright than PE or APC, PerCP performs reliably for low-background targets.
- Alexa Fluor Series: Known for high brightness and excellent photostability, maintaining stable signals under prolonged or repeated measurements. The series covers multiple wavelengths from violet to far-red, offering flexibility in high-dimensional panel design, especially for low-expression antigens or photobleaching-sensitive experiments.
Find the Ideal Fluorophores for Antibody Labeling
| Catalog | Name | CAS | Color | Ex (nm) | Em (nm) | Applications | Inquiry |
|---|---|---|---|---|---|---|---|
| F04-0033 | 5-Aminofluorescein | 3326-34-9 | Green | 495 | 515 | Cell labeling, antibody conjugation, microscopy | Bulk Inquiry |
| F04-0012 | FITC isomer I | 3326-32-7 | Green | 495 | 519 | Protein labeling, flow cytometry, microscopy | Bulk Inquiry |
| F04-0055 | Dexamethasone Fluorescein | 216854-76-1 | Green | 490-495 | 515-520 | Receptor binding assays, drug localization | Bulk Inquiry |
| F04-0026 | Fluorescein-6-isothiocyanate | 18861-78-4 | Green | 495 | 515 | Protein/peptide labeling, flow cytometry | Bulk Inquiry |
| R01-0472 | Atto 425-NHS ester | 892156-28-4 | Blue | 436 | 484 | Confocal microscopy, FRET, labeling of biomolecules | Bulk Inquiry |
| F04-0036 | Fluorescein isothiocyanate-dextran | 60842-46-8 | Green | 495 | 515 | Cell tracing, permeability studies, microscopy | Bulk Inquiry |
| A17-0084 | Fluorescein sodium salt | 518-47-8 | Green | 490 | 514 | General fluorescence assays, microscopy | Bulk Inquiry |
| F04-0027 | Fluorescein-5-maleimide | 75350-46-8 | Green | 494 | 518 | Thiol labeling, protein conjugation | Bulk Inquiry |
| R01-0471 | AF647 NHS ester | 407627-60-5 | Far-red | 650 | 668 | Flow cytometry, immunofluorescence, multicolor panels | Bulk Inquiry |
| R01-0451 | AF 488 TFP ester | 2133404-55-2 | Green | 495 | 519 | Protein labeling, antibody conjugation, flow cytometry | Bulk Inquiry |
Looking for Antibody Labeling Dyes?
We provide flexible conjugation options with various fluorophores, including water-soluble and photostable dyes, to meet your experimental requirements.
Direct vs. Indirect Fluorescent Antibody Labeling in Flow Cytometry
Fluorescent antibody labeling in flow cytometry is mainly classified into direct and indirect approaches. Choosing the appropriate labeling strategy affects experimental sensitivity and signal intensity, as well as cost, workflow, and flexibility in multicolor panel design. Understanding the characteristics and suitable applications of each method is critical for optimizing experimental protocols.
Direct Fluorescent Antibody Labeling
Direct labeling refers to antibodies that are already covalently conjugated to a fluorophore, allowing them to bind directly to target antigens and emit detectable signals.
Characteristics:
- Clear labeling structure and low background: Only a single antibody molecule carries the fluorophore, resulting in relatively low nonspecific binding and background signals.
- Simplified workflow and good reproducibility: Staining is completed without additional steps, reducing operational errors and saving time.
- Suitable for high-throughput experiments: Facilitates rapid screening and handling of multiple samples, particularly useful for immunophenotyping and high-throughput drug or functional screening.
Applications:
- Experiments requiring rapid detection due to time constraints
- Multicolor immunophenotyping
- High-throughput sample analysis
The advantage of direct labeling is operational simplicity and reliable results. However, for low-abundance antigen detection or when signal enhancement is needed, high-brightness fluorophores or indirect labeling may be preferred.
Indirect Fluorescent Antibody Labeling
Indirect labeling uses an unlabeled primary antibody to bind the antigen first, followed by a fluorophore-conjugated secondary antibody that recognizes the primary antibody to generate a signal.
Characteristics:
- Signal amplification: One primary antibody can bind multiple secondary antibodies, significantly enhancing fluorescence signals, especially for low-abundance antigens.
- High flexibility: A single secondary antibody can be used with multiple primary antibodies, saving costs and simplifying fluorophore selection for different experiments.
- Suitable for complex samples: Provides stronger signal contrast when detecting intracellular proteins or rare cell populations.
Considerations:
- Potential for increased background: Excessive or nonspecific secondary antibody binding may cause false positives; titration and blocking conditions must be optimized.
- Strict controls required: Single-stained, unstained, and FMO controls are essential to ensure accurate compensation and reliable data.
- More complex workflow and variability: Multiple staining steps increase operational complexity, requiring good laboratory skills and standardized procedures.
How to Choose the Right Fluorescent-Labeled Antibodies?
| Selection Factor | Considerations | Recommended Strategy | Notes & Examples |
|---|---|---|---|
| Target Antigen Expression | High vs. low expression | High expression → Direct labelingLow expression → Indirect labeling | Low-abundance proteins or rare cell populations can benefit from signal amplification via indirect labeling, e.g., Treg surface markers. |
| Signal Sensitivity | Required fluorescence intensity | Weak or low-abundance antigens → Use indirect labeling or high-brightness fluorophores | High-brightness fluorophores (PE, APC) are ideal for low-expression targets; direct labeling works well for high-expression antigens. |
| Experimental Complexity & Time | High-throughput vs. detailed analysis | Fast, high-throughput → Direct labelingDetailed/multi-step analysis → Indirect labeling | Direct labeling reduces hands-on time, suitable for multi-sample immunophenotyping. |
| Signal Amplification Requirement | Need for enhanced signal? | Low-abundance proteins or intracellular antigens → Indirect labeling | Secondary antibodies amplify signal, enabling detection of rare or weakly expressed molecules. |
| Multicolor Panel Design | Spectral overlap and compensation | Assign high-brightness fluorophores to low-expression antigensAvoid spectral conflicts | Use single-stain and FMO controls to optimize compensation and minimize channel spillover. |
| Instrument Configuration | Laser wavelengths and detection channels | Fluorophores must match instrument laser lines | 488 nm laser → FITC, PE; 633 nm laser → APC; 405 nm laser → BV421, Pacific Blue. |
| Cost & Availability | Antibody and fluorophore cost | Common antigens → Direct labelingRare targets or special applications → Indirect labeling | Secondary antibodies in indirect labeling are reusable across multiple primaries, reducing cost for rare antibodies. |
Balancing experimental objectives, sample characteristics, and instrument configuration helps researchers select the optimal labeling strategy to ensure signal sensitivity, data reliability, and reproducibility.
Applications of Fluorescent-Labeled Antibodies in Flow Cytometry
Fluorescent-labeled antibodies have broad applications in flow cytometry and are indispensable in immunology, oncology, stem cell research, and drug development. By specifically recognizing surface or intracellular antigens and combining high-sensitivity fluorescence detection, researchers can analyze cell population characteristics, functional states, and signaling pathways at the single-cell level. Common applications include:
Immunophenotyping and Cell Population Analysis
Fluorescent-labeled antibodies are central to immunophenotyping, specifically identifying cell surface molecules such as CD3, CD4, CD8, CD19, CD56, etc., to distinguish immune cell subsets like T cells, B cells, NK cells, and monocytes. Multicolor combinations allow simultaneous detection of multiple markers in a single experiment, enabling high-throughput and precise characterization of complex immune populations. This approach is widely used in basic immunology research, clinical immune monitoring, disease diagnosis, and therapeutic assessment, providing reliable data for precision immunology.
Cell Cycle and Apoptosis Detection
Fluorescent-labeled antibodies can analyze cellular functional states, including cell cycle and apoptosis. By labeling cell cycle proteins such as Ki-67 or Cyclin B1 and combining with DNA dyes (e.g., PI or DAPI), researchers can accurately distinguish G0/G1, S, and G2/M phase cells. For apoptosis detection, fluorescent antibodies against Annexin V or activated caspases differentiate early and late apoptotic cells. These applications provide high-sensitivity, single-cell data for drug screening, cellular stress response studies, and cell dynamics analysis.
Intracellular and Surface Protein Quantification
Fluorescent antibodies can detect both surface and intracellular proteins for multiparameter quantitative analysis. Surface proteins, such as immune receptors, inform studies on cell recognition, signaling, and activation, while intracellular proteins like transcription factors or cytokines can be labeled after fixation and permeabilization to analyze signaling pathways or protein abundance. This method is widely applied in functional immune analysis, pathway research, and drug evaluation, providing precise single-cell quantification of protein expression and activity.
Functional Assays for Cytokine and Signaling Pathways
Fluorescent-labeled antibodies assess cytokine production and signaling pathway activation. For example, labeling intracellular IL-2, IFN-γ, or TNF-α enables rapid immune response evaluation, while fluorescent labeling of phosphorylated proteins (e.g., pERK, pAKT, pSTAT) monitors signaling dynamics. Using multicolor flow cytometry, multiple functional parameters can be measured simultaneously, providing high-resolution single-cell data for drug screening, immune function assessment, and signaling mechanism studies.
Experimental Workflow for Using Fluorescent-Labeled Antibodies
Successful flow cytometry applications rely not only on high-quality antibodies and appropriate fluorophore selection but also on a systematic, standardized workflow to ensure accurate and reproducible data. A proper workflow includes sample preparation, cell staining, control setup, data acquisition, and analysis, with each step directly affecting experimental outcomes.
1. Sample Preparation
- Cell source: Peripheral blood mononuclear cells (PBMCs), tissue-isolated cells, or cell lines.
- Single-cell suspension: Remove aggregates and debris via filtration or centrifugation to ensure smooth flow in the cytometer.
- Cell counting and viability assessment: Use trypan blue or automated counters to maintain appropriate cell concentration (typically 0.5–1×10^6/mL) and high viability.
2. Cell Staining
- Surface antigen staining: Directly add labeled antibodies to cell suspensions and incubate gently for specific binding.
- Intracellular antigen staining: Fixation and permeabilization are essential; commercial kits can preserve cellular structure while allowing antibody access.
- Fluorescent secondary antibody staining (indirect): Add unlabeled primary antibody first, then fluorophore-conjugated secondary antibody to amplify signal.
- Staining optimization: Adjust antibody concentration and incubation time to avoid nonspecific binding or signal quenching.
3. Controls:
- Single-stained controls: For compensation and correction of fluorescence spillover.
- Fluorescence Minus One (FMO) controls: Aid in setting gating boundaries to avoid misclassification.
- Unstained controls: Assess background fluorescence and instrument noise.
- Assign high-brightness fluorophores to low-abundance antigens to minimize spectral overlap and complex compensation.
- Consider laser excitation wavelengths, detector configuration, and fluorophore spectral properties to maximize signal resolution.
- Use FSC/SSC (forward/side scatter) to differentiate cell populations and exclude debris or dead cells.
- Stepwise gating: select live cells, identify major subsets, and finally analyze target marker expression.
- Ensure cytometer is calibrated and QC-verified.
- Set the number of events to acquire: 10,000–50,000 events for routine analysis; higher for rare populations.
- Use professional software (e.g., FlowJo, FCS Express).
- Correct spectral spillover using compensation matrices for accurate multicolor data.
- Quantify fluorescence intensity, population proportions, and expression levels to support downstream functional or mechanistic studies.
FITC-Conjugated Antibodies
- We offer FITC (Fluorescein Isothiocyanate) conjugation services to meet both research and clinical needs.
- Efficient covalent labeling is available for proteins, monoclonal, and polyclonal antibodies.
- Supports multicolor flow cytometry and immunofluorescence imaging applications.
- Optimized conjugation conditions are provided to ensure signal stability and high specificity.
PE-Conjugated Antibodies
- We provide PE (Phycoerythrin) conjugation services, ideal for multicolor immunoassays.
- Efficient covalent labeling for antibodies and proteins ensures exceptional signal intensity.
- Supports complex immunophenotyping and high-throughput flow cytometry experiments.
- Optimized conjugation protocols guarantee high brightness with minimal background.
APC-Conjugated Antibodies
- APC (Allophycocyanin) conjugation services are available, compatible with multichannel flow detection.
- Efficient fluorescent labeling for monoclonal and polyclonal antibodies.
- Far-red fluorescence reduces background interference, ideal for high-content imaging and multicolor experiments.
- Optimized conjugation conditions ensure clear and stable signals.
Alexa Fluor Series Conjugated Antibodies
- High-brightness Alexa Fluor conjugation services are provided for precise imaging applications.
- Efficient covalent labeling is available for proteins, antibodies, and nanomaterials.
- Supports multicolor immunofluorescence staining and high-content analysis platforms.
- Optimized conjugation conditions enhance photostability, ensuring reliable and stable signals.
4. Multicolor Panel Design:
5. Gating Strategy:
6. Data Acquisition:
7. Data Analysis:
Common Challenges and Troubleshooting Tips
Even when using high-quality antibodies and optimized fluorophore combinations, various technical challenges may arise during flow cytometry experiments with fluorescent-labeled antibodies. Identifying the source of problems and implementing targeted solutions is essential to ensure data accuracy and reproducibility. The following are common issues and recommended strategies.
Reducing Non-Specific Binding and Background Fluorescence
Non-specific binding and high background fluorescence are among the most frequent issues in flow cytometry, potentially leading to false positives or poorly resolved subpopulation signals. Non-specific binding is often mediated by Fc receptors or caused by excessive antibody concentration, dead cells, or sample impurities. To reduce background signals, use Fc blockers or appropriate blocking buffers prior to staining and optimize antibody concentration through titration. Maintaining cell viability and integrity, and removing dead cells and debris, also significantly decreases background interference, yielding clearer and more reliable signals.
Solving Fluorescence Spillover and Compensation Errors
In multicolor flow cytometry experiments, fluorescence spillover is another major challenge. When the emission spectra of different fluorophores overlap, signals can bleed into adjacent channels, causing measurement errors. Spillover issues are often associated with improper panel design, inaccurate compensation matrix setup, or insufficient single-stained controls. To address this problem, carefully select fluorophore combinations and avoid using dyes with severe spectral overlap in the same panel. Additionally, include single-stained controls for each fluorophore to establish an accurate compensation matrix and use FMO controls to define gating boundaries. Regular calibration and performance checks of the instrument ensure that detectors and optical systems remain stable, improving compensation accuracy and the reliability of multicolor analyses.
Ensuring Batch-to-Batch Consistency and Reproducibility
Batch variation and reproducibility are especially critical in repeated experiments or long-term projects. Differences between antibody lots or uneven fluorophore conjugation can lead to signal variability, while inconsistent workflows or instrument settings may further increase variation. To ensure consistency, choose high-quality antibodies with strict QC validation and, whenever possible, use the same lot throughout the study. Standardizing experimental procedures—including staining time, temperature, antibody concentration, and washing steps—is essential to minimize operator-induced variability. Instrument maintenance, including regular calibration and quality control, ensures stable light sources, detectors, and flow rates. Finally, detailed documentation of experimental parameters and procedures helps with troubleshooting and reproducibility, guaranteeing reliable and consistent data.
Why Choose BOC Sciences for Fluorescent-Labeled Antibodies?
High-quality fluorescent-labeled antibodies are essential for successful flow cytometry experiments. BOC Sciences provides comprehensive, customizable solutions to meet diverse needs from basic research to preclinical development. Our advantages span antibody quality, fluorophore variety, customization capabilities, and multicolor panel support.
Do You Need A Consultation?
BOC Sciences integrates cutting-edge fluorescence technologies to accelerate your research, driving next-generation solutions for drug discovery and diagnostics.
References
- Vira S, et al. Fluorescent-labeled antibodies: Balancing functionality and degree of labeling. Anal Biochem. 2010; 402(2):146–150. DOI: 10.1016/j.ab.2010.03.036. PMID: 20362543.
- Pitsillides CM, et al. Cell labeling approaches for fluorescence-based in vivo flow cytometry. Cytometry A. 2011; 79(10):758–765. DOI: 10.1002/cyto.a.21125. PMID: 21905206.
- Holmes K, et al. Preparation of cells and reagents for flow cytmetry. Curr Protoc Immunol. 2001; Chapter 5:Unit 5.3. DOI: 10.1002/0471142735.im0503s44. PMID: 18432799.
Transform Your Studies with Cutting-Edge Fluorescent Products
| Catalog | Name | CAS | Inquiry |
|---|---|---|---|
| F04-0033 | 5-Aminofluorescein | 3326-34-9 | Bulk Inquiry |
| A16-0170 | Rhodamine-123 | 62669-70-9 | Bulk Inquiry |
| A19-0040 | Hoechst 33342 | 23491-52-3 | Bulk Inquiry |
| F06-0011 | Coumarin 153 | 53518-18-6 | Bulk Inquiry |
| F02-0026 | Cy5-NHS ester | 146368-14-1 | Bulk Inquiry |
| F01-0221 | BODIPY Green 8-P2M | 929679-22-1 | Bulk Inquiry |
| A16-0201 | DAPI dihydrochloride | 28718-90-3 | Bulk Inquiry |
| A17-0186 | Perylene Orange | 82953-57-9 | Bulk Inquiry |
| A19-0103 | SYBR Green I | 178918-96-2 | Bulk Inquiry |
| F01-0251 | BODIPY 576/589 | 150173-78-7 | Bulk Inquiry |
High-Performance Fluorescent Tools for Your Research
- TAMRA Dyes Red-emitting dyes for antibody and protein labeling.
- ATTO Dyes High-performance dyes for labeling and imaging experiments.
- Coumarin Blue-emitting dyes for chemical sensing and fluorescence studies.
- Fluorescent Protein Used for live-cell imaging and real-time biosensing.
More About Antibody labeling
Online Inquiry

