Tips for Reliable Imaging with Fluorescent-Labeled Antibodies in Immunofluorescence
Fluorescent-labeled antibodies are one of the most essential tools in immunofluorescence microscopy. By conjugating specific antibodies with high-performance fluorophores, researchers can precisely localize target proteins within cellular and tissue environments and obtain clear, reproducible imaging data. As microscopy technologies continue to advance, immunofluorescence not only enables high-resolution spatial visualization but also supports multiplex detection, dynamic process tracking, and in-depth molecular analysis. However, achieving high-quality imaging is not always straightforward—fluorophore selection, antibody specificity, sample preparation methods, and imaging parameters all influence the clarity and reliability of the final images. To help researchers obtain more stable, brighter, and highly interpretable results, this guide systematically explains the key principles of fluorescent-labeled antibodies, best experimental practices, and strategies to resolve common issues. Whether you are conducting fundamental cell biology research or focusing on drug development and disease mechanism studies, understanding these core elements will significantly enhance the success rate and data quality of immunofluorescence experiments.
Fluorescent-Labeled Antibodies in Immunofluorescence
Fluorescent-labeled antibodies are the central tools of immunofluorescence microscopy, capable of binding specifically to target proteins or molecules and converting molecular information into visible fluorescent signals. In cell biology, molecular research, and pathology, fluorescent-labeled antibodies provide researchers with the ability to observe cellular structures, protein distribution, and dynamic changes. Understanding their principles and mechanisms is the first step in designing high-quality immunofluorescence experiments.
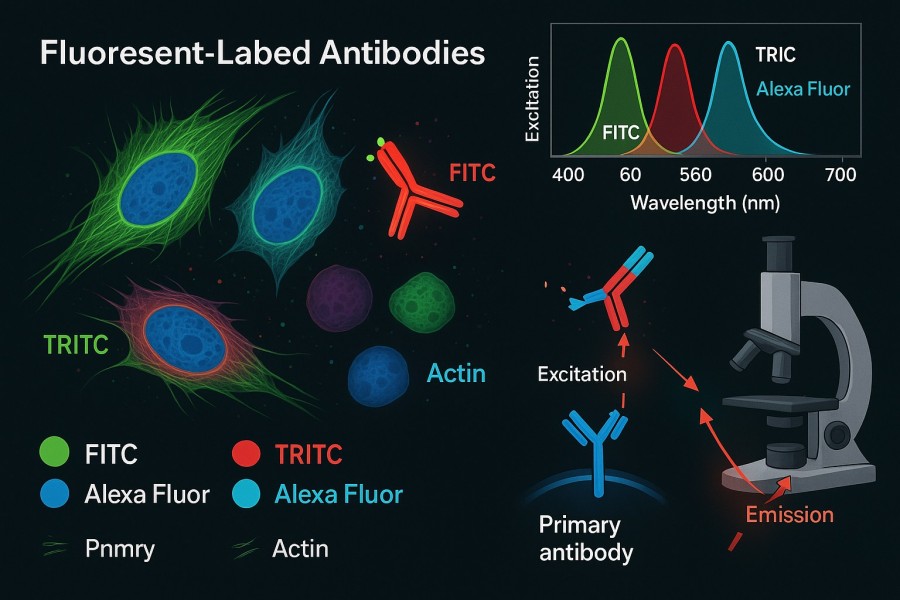 Fig. 1. Fluorescent-labeled antibodies in immunofluorescence (BOC Sciences Authorized).
Fig. 1. Fluorescent-labeled antibodies in immunofluorescence (BOC Sciences Authorized).
How Fluorescent Antibodies Bind to Target Proteins?
Fluorescent-labeled antibodies recognize target molecules through antigen–antibody specific interactions, mainly in two forms: direct labeling and indirect labeling.
Direct Labeling
In direct labeling, fluorophores are covalently conjugated to the primary antibody, generating a detectable signal as soon as the antibody binds to the target protein. This approach has advantages such as simplified workflow, reduced experimental time, and minimized background signal from secondary antibodies. However, the limited number of fluorophores per antibody may result in slightly lower signal intensity, particularly when detecting low-abundance proteins.
Indirect Labeling
Indirect labeling uses an unlabeled primary antibody to recognize the target protein, followed by a fluorophore-labeled secondary antibody that binds to the primary antibody's Fc region, thereby amplifying the signal. The advantages include:
- Signal enhancement: A single primary antibody can be recognized by multiple secondary antibodies, significantly increasing fluorescence intensity.
- High flexibility: The same secondary antibody can be used with various primary antibodies, reducing experimental costs.
- Convenient multicolor imaging: By selecting secondary antibodies labeled with different fluorophores, multiple targets can be detected simultaneously.
During binding, the affinity and specificity of the antibody are key factors determining imaging precision and signal-to-noise ratio. High-affinity antibodies bind tightly to target proteins, while highly specific antibodies minimize background noise caused by nonspecific binding.
Advantages of Using Fluorescent Labels for Live and Fixed Cells
Fluorescent-labeled antibodies offer significant advantages over traditional staining methods, making them indispensable tools in cell biology research:
- High-sensitivity detection: Fluorophores provide excellent optical sensitivity, enabling reliable detection of trace proteins or low-abundance molecules.
- Spatial localization capability: Fluorescent signals precisely reveal protein distribution in organelles, membrane structures, and intra- or extracellular locations, providing a solid foundation for studying cellular architecture.
- Dynamic tracking and real-time monitoring: In live-cell imaging, fluorescent antibodies allow real-time monitoring of protein trafficking, signal transduction, and cell behavior, providing time-resolved functional insights.
- Multiplex labeling and colocalization studies: Multiple fluorescent antibodies can be applied simultaneously to different targets, enabling colocalization analysis with multichannel microscopy and revealing protein interactions and signaling networks.
- Quantitative analysis: Fluorescence intensity measurements allow semi-quantitative or quantitative assessment of protein expression levels, offering reliable data for studying molecular mechanisms.
Key Applications in Cell Biology and Molecular Research
Signaling Pathways and Protein Dynamics: Fluorescent-labeled antibodies play an important role in studying signaling pathways and protein dynamics. Researchers can track the intracellular movement and activation of transcription factors, enzymes, or receptors, thereby analyzing activation rates and time windows of signaling pathways. This dynamic tracking capability reveals subtle changes in intracellular signaling and provides quantitative insights into molecular mechanisms.
Organelle and Protein Localization: Fluorescent antibodies help identify protein distribution in specific cell structures such as the nucleus, mitochondria, endoplasmic reticulum, or cell membrane. Combined with high-resolution or super-resolution microscopy, fluorescent antibodies reveal fine subcellular architecture and interactions between proteins and organelles, offering reliable visual evidence for understanding cellular function.
Pathology and Histology: In tissue-section analysis, fluorescent-labeled antibodies can detect disease-associated molecules such as tumor markers or neural proteins. Through immunofluorescence imaging, researchers can visualize molecular distribution and abnormalities at the tissue level, supporting disease diagnosis and pathology research with intuitive data.
Drug Research and Screening: Fluorescent antibodies can assess changes in protein expression and localization following drug treatment, providing direct evidence for drug mechanism studies. With high-throughput imaging platforms, large sample sets can be analyzed in a short time, enhancing efficiency and accuracy in drug screening and pharmacological evaluation.
Multimodal Imaging and Functional Studies: Fluorescent antibodies can be combined with fluorescent proteins, functional dyes, or nanoprobes to enable multiplex and multi-parameter monitoring. This allows comprehensive analysis of cell behavior, protein interactions, and signal dynamics, providing a more complete information map for understanding complex cellular functions and molecular networks.
Choosing the Right Fluorophore for Clear Fluorescence Imaging
The choice of fluorophore directly determines the clarity and signal-to-noise ratio of immunofluorescence imaging. Different fluorescent dyes have distinct excitation and emission spectra, brightness, and photostability. Selecting fluorophores compatible with microscope settings significantly enhances image quality while reducing background interference.
Matching Excitation and Emission Spectra to Your Microscope
When selecting a fluorophore, the excitation and emission wavelengths must be compatible with the microscope's light source and filter sets. The excitation spectrum must match the illumination source for efficient excitation, while the emission spectrum must align with the detector's sensitivity. Mismatched spectra can result in reduced signal intensity and blurred images. Additionally, in multicolor imaging, fluorophores must have well-separated spectra to avoid signal crosstalk and channel overlap, ensuring independent detection of each target.
Brightness and Photostability Considerations
Fluorophore brightness and photostability are critical for high-quality imaging. Highly bright fluorophores generate clear signals with short exposure times, reducing photobleaching risks. Highly photostable fluorophores maintain signal strength during long-term or dynamic imaging, preventing signal decay caused by light exposure. Researchers should evaluate experimental duration, sample sensitivity, and microscope performance to select fluorophores that ensure reliable imaging outcomes.
Multi-Color Imaging and Avoiding Spectral Overlap
In experiments requiring simultaneous observation of multiple targets, multicolor immunofluorescence is a common strategy. When choosing fluorophores, their spectral separation must be considered to avoid overlapping emission signals that cause interference. Fluorophores should also align with microscope filter configurations to enable independent signal detection. Properly pairing fluorophores enhances imaging accuracy and supports colocalization analysis, protein interaction studies, and dynamic monitoring of complex cellular behaviors.
Experimental Optimization Strategies for Fluorophore Selection
In practice, researchers can conduct preliminary tests to compare fluorophores under specific cell types and microscopy conditions. Signal intensity, background noise, and photobleaching rates can be evaluated to determine the optimal fluorophore combination. Additionally, considering the chemical stability and conjugation efficiency of fluorophores ensures that labeled antibodies remain reliable during storage and long-term experiments.
Common Fluorophores for Fluorescent-Labeled Antibodies
The performance of fluorescent-labeled antibodies largely depends on the chosen fluorophore. Different fluorophores vary in excitation and emission wavelengths, brightness, photostability, and compatibility with microscopes. Understanding the characteristics and application scenarios of commonly used fluorophores helps researchers make informed selections based on experimental needs, ensuring high-quality, clear, and reliable immunofluorescence imaging results.
FITC (Fluorescein Isothiocyanate)
FITC is a classic green fluorophore with an excitation wavelength of approximately 495 nm and an emission wavelength of about 519 nm. It offers moderate brightness and average photostability, making it suitable for fixed cells and short-term imaging. FITC-conjugated antibodies are widely used in single- or multi-color immunofluorescence experiments, though spectral overlap with other green or yellow-green fluorophores should be avoided in multi-color imaging.
Alexa Fluor Series
Alexa Fluor dyes are high-brightness, highly photostable fluorophores, including Alexa Fluor 488, 555, 594, 647, and others. Their excitation and emission wavelengths span from green to far-red regions, making them ideal for multi-color and long-term imaging. Alexa Fluor-labeled antibodies perform well in both live and fixed cell experiments, effectively reducing photobleaching while providing strong signals. They are among the preferred fluorophores for modern immunofluorescence applications.
Cy Series (Cy3, Cy5)
Cy dyes exhibit bright red to far-red fluorescence (e.g., Cy3 Ex 550 nm / Em 570 nm; Cy5 Ex 650 nm / Em 670 nm) with good photostability. The Cy series is commonly used in multi-color colocalization experiments and is especially suitable when combined with green fluorophores, enabling expanded multiplex imaging capabilities. Their far-red spectra also help reduce interference from cellular autofluorescence.
TRITC (Tetramethylrhodamine Isothiocyanate)
TRITC is another widely used red fluorophore, with an excitation wavelength of approximately 550 nm and an emission wavelength of about 570 nm. It offers moderate brightness and relatively low photostability, making it suitable for short-term observation and fixed cell experiments. TRITC can be paired with FITC for dual-color imaging, though care must be taken to avoid photobleaching and spectral overlap.
DAPI (4',6-Diamidino-2-Phenylindole)
DAPI is a blue fluorescent dye with an excitation wavelength of approximately 358 nm and an emission wavelength of about 461 nm. It is commonly used for nuclear staining and can be combined with other fluorophore colors for multi-color immunofluorescence. DAPI has high photostability and produces clear signals, making it a standard choice for nuclear labeling and colocalization studies.
Table. 1. Fluorescent reagents from BOC Sciences.
| Catalog | Name | CAS | Color | Ex (nm) | Em (nm) | Applications | Inquiry |
|---|---|---|---|---|---|---|---|
| A17-0083 | Cy7-NHS ester | 477908-53-5 | Near-IR | ~750 | ~776 | Antibody labeling, in vivo imaging, far-red multiplex IF | Bulk Inquiry |
| A01-0005 | Rhodamine B | 81-88-9 | Red | ~554 | ~576 | Cell staining, fluorescence microscopy, tracer studies | Bulk Inquiry |
| A16-0142 | Dihydrorhodamine 6G | 217176-83-5 | Green-Yellow | ~528 | ~551 | ROS detection, flow cytometry, live-cell assays | Bulk Inquiry |
| F04-0037 | Fluorescein | 2321-07-5 | Green | ~494 | ~521 | Immunofluorescence, nucleic acid labeling, microscopy | Bulk Inquiry |
| F04-0007 | Fluorescein-Maleimide | 2228857-33-6 | Green | ~494 | ~521 | Thiol-reactive labeling, protein conjugation, IF | Bulk Inquiry |
| R01-0471 | AF647 NHS ester | 407627-60-5 | Far-Red | ~650 | ~668 | High-sensitivity IF, flow cytometry, multi-color imaging | Bulk Inquiry |
| R01-0039 | AF430 NHS ester | 467233-94-9 | Blue | ~430 | ~544 | Protein labeling, microscopy, FRET applications | Bulk Inquiry |
| R01-0044 | AF488 azide | 1679326-36-3 | Green | ~495 | ~519 | Click chemistry labeling, IF, flow cytometry | Bulk Inquiry |
| A03-0016 | Oregon Green 488 carboxylic acid | 195136-52-8 | Green | ~496 | ~524 | pH-insensitive labeling, microscopy, fluorescence assays | Bulk Inquiry |
| F03-0017 | Sulfo-Cyanine7 maleimide | 2183440-60-8 | Near-IR | ~750 | ~773 | Thiol labeling, in vivo imaging, far-red IF | Bulk Inquiry |
Looking for Antibody Labeling Dyes?
We provide flexible conjugation options with various fluorophores, including water-soluble and photostable dyes, to meet your experimental requirements.
Best Practices for Antibody Selection and Optimization
In immunofluorescence experiments, antibody selection and optimization are critical to achieving clear imaging and reliable signals. Improper antibody selection or usage conditions may lead to non-specific signals, insufficient signal intensity, or experimental failure. Therefore, selecting appropriate primary and secondary antibodies, validating antibody specificity, and optimizing concentration and incubation conditions are essential prerequisites for high-quality immunofluorescence imaging.
Primary vs. Secondary Antibody Choices
Primary antibodies directly recognize the target protein, and their specificity and affinity determine the fundamental signal quality. When selecting primary antibodies, researchers should prioritize well-validated research-grade antibodies and refer to published data or vendor-provided validation results. The host species of the primary antibody must also be considered to ensure proper pairing with secondary antibodies.
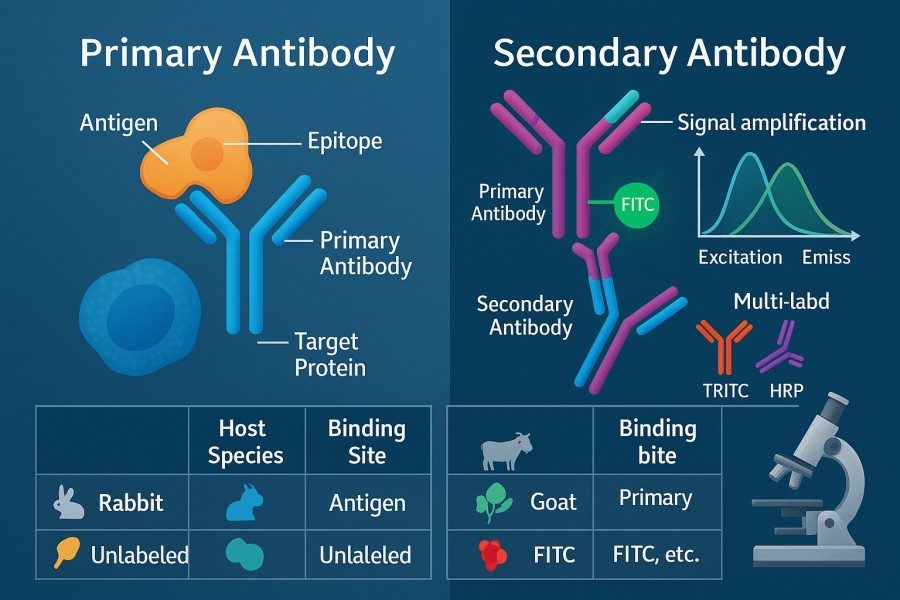 Fig. 2. Primary vs. secondary antibody (BOC Sciences Authorized).
Fig. 2. Primary vs. secondary antibody (BOC Sciences Authorized).
Secondary antibodies typically carry fluorophore labels and amplify the signal indirectly. The species of the primary and secondary antibodies must match—for example, a rabbit primary antibody should be paired with an anti-rabbit secondary antibody. Indirect labeling enhances signal intensity and offers flexibility for multi-color imaging and repeated use of the same secondary antibody. In multiplex experiments, selecting secondary antibodies labeled with different fluorophores and aligning them with the microscope's filter configuration helps avoid spectral overlap and ensures independent signal detection.
Validating Antibody Specificity
Validating antibody specificity is essential for experimental success. Even antibodies from reputable suppliers may behave differently across cell types or fixation conditions. Researchers should validate specificity using positive controls (samples known to express the target protein) and negative controls (samples lacking the target protein). Pre-absorption tests or siRNA knockdown approaches can also help confirm that the observed fluorescence signal accurately reflects the target protein distribution.
Optimizing Antibody Concentration and Incubation Time
Antibody concentration and incubation conditions significantly influence imaging results. Excessive concentrations may cause non-specific binding and increase background noise, whereas insufficient concentrations may produce weak or undetectable signals. Gradient titration is recommended to determine the optimal concentration. Incubation temperature and duration must also be optimized: short incubations at room temperature may be suitable for some membrane proteins, whereas overnight incubation at 4°C is often preferred for low-abundance or difficult-to-bind targets. Optimization should consider cell type, antigen characteristics, and fluorophore properties.
Antibody Storage and Reuse Strategies
Fluorescent-labeled antibodies are sensitive to storage conditions. Maintaining high experimental quality requires preserving antibody activity throughout storage and use. Antibodies should be stored at low temperatures and protected from light, while repeated freeze-thaw cycles should be avoided. For multiple uses of the same antibody, aliquoting diluted stocks or adding preservatives can extend shelf life while maintaining signal stability.
Sample Preparation Tips to Enhance Fluorescence Signal
In immunofluorescence experiments, even high-quality fluorescent antibodies may produce weak signals, high background, or uneven staining if samples are not properly prepared. Sample preparation includes fixation, permeabilization, blocking, and washing—each step directly influencing fluorescence quality. Mastering proper sample preparation techniques maximizes antigen preservation, reduces non-specific fluorescence, and ensures clear and reliable imaging.
Fixation Methods That Preserve Antigenicity
Fixation is essential for preserving cellular structure and antigen epitopes. Common fixatives include paraformaldehyde, ethanol, and methanol:
- Paraformaldehyde fixation: A gentle method that preserves cell morphology and most antigenic sites. Suitable for most cellular proteins and intracellular structures. Typically, 4% paraformaldehyde is used at room temperature for 10–20 minutes, followed by PBS washes to remove residual fixative.
- Ethanol/methanol fixation: Suitable for membrane proteins or nuclear proteins but may denature some intracellular epitopes. Low-temperature fixation—often at –20°C for 5–10 minutes—provides better results.
- Combined fixation: For specific proteins or complex structures, paraformaldehyde fixation followed by ethanol permeabilization can balance structural preservation with antigen exposure.
Fixation strategies should be optimized based on target protein characteristics, cell type, and experimental requirements. Small-scale testing is recommended to ensure strong and uniform fluorescence signals.
Permeabilization Techniques for Intracellular Targets
For intracellular or nuclear proteins, antibodies must pass through the cell membrane. Permeabilization can be achieved using surfactants or chemical agents:
- Triton X-100 is a commonly used non-ionic surfactant that effectively disrupts cellular membranes and is suitable for many proteins and cell types.
- Saponin temporarily permeabilizes the membrane while preserving membrane structure and organelle integrity.
- Digitonin is often used for selective permeabilization of mitochondria or specific cytoplasmic regions.
Permeabilization conditions (concentration, temperature, duration) must be optimized according to protein localization and experimental needs. Over-permeabilization may damage cellular structures and reduce signal quality, whereas insufficient permeabilization may prevent antibodies from adequately binding to their targets.
Reducing Background Fluorescence for Cleaner Images
Non-specific binding reduces the signal-to-noise ratio and affects image clarity. The blocking step is essential for minimizing background signals:
- Choice of blocking buffer: Commonly used PBS buffers containing BSA, normal serum, or non-specific proteins can effectively cover potential binding sites.
- Blocking time and temperature: Incubate at room temperature for 30–60 minutes or overnight at 4°C, and adjust according to experimental needs.
- Washing steps: After blocking, wash thoroughly to remove free blocking proteins or residual impurities to avoid interference with fluorescence signals.
In addition, low-autofluorescence media, slides, or carriers should be used whenever possible to prevent background fluorescence originating from the sample itself.
Sample Storage and Anti-Photobleaching Treatments
Fluorescence signals are highly susceptible to photobleaching. Proper sample handling can extend imaging time and maintain signal stability:
- Use mounting media containing anti-fading reagents (such as ProLong or Vectashield) to reduce photobleaching.
- Avoid prolonged exposure to strong light; use low-intensity, rapid-imaging strategies during microscope operation.
- For long-term storage, keep samples at low temperature and protected from light to prevent fluorophore degradation or antibody inactivation.
Common Challenges in Immunofluorescence and How to Overcome Them
Even when high-quality antibodies are selected and sample preparation is optimized, immunofluorescence experiments may still face a variety of technical challenges, such as non-specific binding, photobleaching, weak signals, or uneven signal distribution. These issues not only affect image clarity but may also distort experimental data. By identifying common problems and applying targeted optimization strategies, researchers can significantly improve experimental success rates and obtain reproducible, reliable imaging results.
Minimizing Non-Specific Binding
Non-specific binding is one of the most frequent issues in immunofluorescence, manifested as increased background or incorrect localization. Solutions include:
- Optimization of blocking: Use blocking buffers containing BSA or normal serum before antibody incubation to sufficiently block non-specific binding sites and reduce background noise.
- Control of antibody concentration and incubation time: Excessive concentration or overly long incubation can cause non-specific binding. Gradient experiments should be conducted to determine optimal conditions.
- Antibody pre-adsorption: For problematic background signals, pre-adsorption can remove antibody molecules that bind to non-target proteins.
- Selection of highly specific antibodies: Prioritize validated primary antibodies, especially for multiplex experiments, to significantly reduce the likelihood of cross-reactivity.
These measures help ensure that fluorescence signals mainly originate from target proteins, improving the signal-to-noise ratio and quantitative reliability.
Handling Photobleaching During Imaging
Photobleaching is a common issue in fluorescence imaging, particularly during dynamic tracking and long-term imaging, where it rapidly weakens signals. Effective strategies include:
- Use of anti-fade mounting media: Products like ProLong and Vectashield can significantly delay fluorescence decay.
- Reducing excitation intensity and exposure time: Use low-intensity, rapid-imaging modes and plan the imaging sequence carefully.
- Choosing photostable fluorophores: During experimental design, prioritize dyes with high photostability, such as Alexa Fluor series.
- Avoiding repeated excitation: For samples requiring multiple observations, acquire the best single image to minimize cumulative photobleaching.
These strategies maintain signal intensity while extending observation time, particularly useful for live-cell dynamic imaging.
Troubleshooting Weak or Uneven Signals
Weak or uneven fluorescence signals significantly affect image quality and are often caused by insufficient antibody binding, improper fixation/permeabilization, or inappropriate microscope settings. Optimization methods include:
- Adjusting antibody concentration and incubation parameters: Use gradient experiments to determine optimal conditions and ensure adequate binding to target proteins.
- Optimizing fixation and permeabilization: Excessive fixation can mask epitopes, whereas insufficient permeabilization may restrict antibody entry. Both must be tailored to the target protein type.
- Checking microscope settings: Verify excitation intensity, exposure time, and gain settings to ensure efficient signal capture without overexposure or noise.
- Confirming antibody batch and storage conditions: Antibody activity may vary between batches, and expired or improperly stored antibodies may cause reduced signals.
By following these troubleshooting steps, researchers can systematically resolve weak or uneven signals to ensure reliable experimental data and interpretable images.
Other Common Issues and Optimization Tips
In addition to the major issues above, immunofluorescence experiments may encounter spectral crosstalk, dye autofluorescence, or interference in multiplex staining. Effective approaches include:
- Rational design of multicolor experiments: Optimize single-color experiments first, then combine them for multiplex labeling to avoid spectral overlap.
- Use of low-autofluorescence materials: Choose low-background slides, media, and mounting reagents to reduce autofluorescence.
- Utilization of image processing and analysis software: Appropriate background subtraction and signal enhancement can improve imaging quality and quantitative accuracy.
Why Choose BOC Sciences for Fluorescent-Labeled Antibodies?
BOC Sciences offers a comprehensive fluorescence chemistry platform and antibody engineering system to provide high-quality fluorescent-labeled antibodies and related solutions for research institutes, pharmaceutical enterprises, and diagnostic companies. From fluorophore development to antibody conjugation, and from experimental design to quality validation, we deliver complete, customizable, and internationally compliant technical services to ensure that researchers obtain highly sensitive, highly specific, and highly reproducible immunofluorescence data.
Fluorophore Synthesis and Modification Services
- Capable of synthesizing commonly used fluorophores such as FITC, Cy dyes, and Alexa Fluor analogs, with customizable spectral properties and structural modifications upon request.
- Provide the introduction of reactive groups (e.g., NHS ester, maleimide, azide) to enhance conjugation efficiency and stability between fluorophores and antibodies.
- Equipped with process scaling and high-purity production capabilities to ensure stable fluorophore performance and excellent batch-to-batch consistency.
- Offer fluorophore recommendations tailored to application scenarios (live-cell imaging, multicolor imaging, long-term tracking).
Custom Fluorescent Antibody Conjugation
- Support fluorescent labeling of various antibody types, including IgG, monoclonal antibodies, polyclonal antibodies, VHH, and recombinant antibodies.
- Provide degree-of-labeling (DOL) optimization with precise control to balance signal intensity and antigen-binding activity.
- Use mild conjugation conditions and proprietary buffer systems to maximize antibody activity and structural stability.
- Comprehensive desalting, purification, and free-dye removal processes ensure low background and clean signals in labeled antibodies.
One-Stop Immunofluorescence Microscopy Solutions
- Offer pre-experiment consultation, including antibody selection, fluorophore combination, and recommendations for fixation and permeabilization methods.
- Provide optimized sample preparation, staining workflows, and working concentrations for complex samples such as tissue sections and multi-target cell lines.
- Support multicolor immunofluorescence design, including spectral matching analysis and strategies to avoid crosstalk.
- Provide signal optimization and troubleshooting assistance to address common issues such as high background, photobleaching, and uneven signals.
Quality Assurance and Analytical Validation
- Perform purity testing, labeling degree measurement, and binding activity validation to ensure labeled antibodies meet experimental requirements.
- Use HPLC, UV-Vis, SDS-PAGE, and fluorescence spectroscopy for multidimensional quality control.
- Provide complete COA (certificate of analysis), experimental parameters, storage conditions, and usage recommendations to support publications and downstream research.
- Maintain a batch traceability system to ensure consistency and reliability across batches for large-scale projects.
Do You Need A Consultation?
BOC Sciences integrates cutting-edge fluorescence technologies to accelerate your research, driving next-generation solutions for drug discovery and diagnostics.
References
- Vira S, et al. Fluorescent-labeled antibodies: Balancing functionality and degree of labeling. Anal Biochem. 2010; 402(2):146–150. DOI: 10.1016/j.ab.2010.03.036. PMID: 20362543.
- Im K, et al. An Introduction to Performing Immunofluorescence Staining. Methods Mol Biol. 2019; 1897:299–311. DOI: 10.1007/978-1-4939-8935-5_26. PMID: 30539454.
- Moreno V, et al. Fluorescent Immunohistochemistry. Methods Mol Biol. 2022; 2422:131–146. DOI: 10.1007/978-1-0716-1948-3_9. PMID: 34859403.
Transform Your Studies with Cutting-Edge Fluorescent Products
| Catalog | Name | CAS | Inquiry |
|---|---|---|---|
| R10-0005 | 6-Fluorescein phosphoramidite | 204697-37-0 | Bulk Inquiry |
| A19-0040 | Hoechst 33342 | 23491-52-3 | Bulk Inquiry |
| A16-0201 | DAPI dihydrochloride | 28718-90-3 | Bulk Inquiry |
| A19-0060 | Hoechst 34580 | 23555-00-2 | Bulk Inquiry |
| A19-0034 | DAPI dilactate | 28718-91-4 | Bulk Inquiry |
| A16-0093 | Rhodamine 6G | 989-38-8 | Bulk Inquiry |
| A19-0041 | Hoechst 33258 | 23491-45-4 | Bulk Inquiry |
| F04-0019 | Fluorescein-PEG5-NHS ester | 2353409-62-6 | Bulk Inquiry |
| R01-0019 | Cyanine5 NHS ester | 350686-88-3 | Bulk Inquiry |
| F02-0012 | Cyanine5.5 carboxylic acid | 1144107-80-1 | Bulk Inquiry |
High-Performance Fluorescent Tools for Your Research
- TAMRA Dyes Red-emitting dyes for antibody and protein labeling.
- ATTO Dyes High-performance dyes for labeling and imaging experiments.
- Coumarin Blue-emitting dyes for chemical sensing and fluorescence studies.
- Fluorescent Protein Used for live-cell imaging and real-time biosensing.
More About Antibody labeling
Online Inquiry

