Fluorescent Antibodies in High-Throughput Screening: Techniques and Best Practices
High-throughput screening (HTS) is a core technology in drug discovery, molecular target validation, and biological pathway research. With the advancement of automation, miniaturized workflows, and detection technologies, fluorescent-labeled antibodies have become increasingly important in HTS. Thanks to their high sensitivity, specificity, and multiplexing capabilities, they are key tools for enhancing screening efficiency, data quality, and imaging resolution. This article provides a comprehensive overview—from technical principles, screening workflows, and fluorophore selection to applications, optimization strategies, and challenges—to help researchers and pharmaceutical companies establish reliable, reproducible, and scalable HTS systems.
What are Fluorescent-Labeled Antibodies?
Fluorescent-labeled antibodies are among the most widely used and essential functional biological reagents in modern bioanalysis and high-throughput screening systems. They are formed by covalently linking a specific antibody, which recognizes the target protein, with a fluorophore capable of emitting fluorescence. In detection, the antibody provides structural specificity, while the fluorophore generates a visible and quantifiable signal. With high sensitivity, specificity, multiplexing ability, and strong compatibility with automated HTS platforms, fluorescent-labeled antibodies drive advancements in genomics, proteomics, drug discovery, and phenotypic screening.
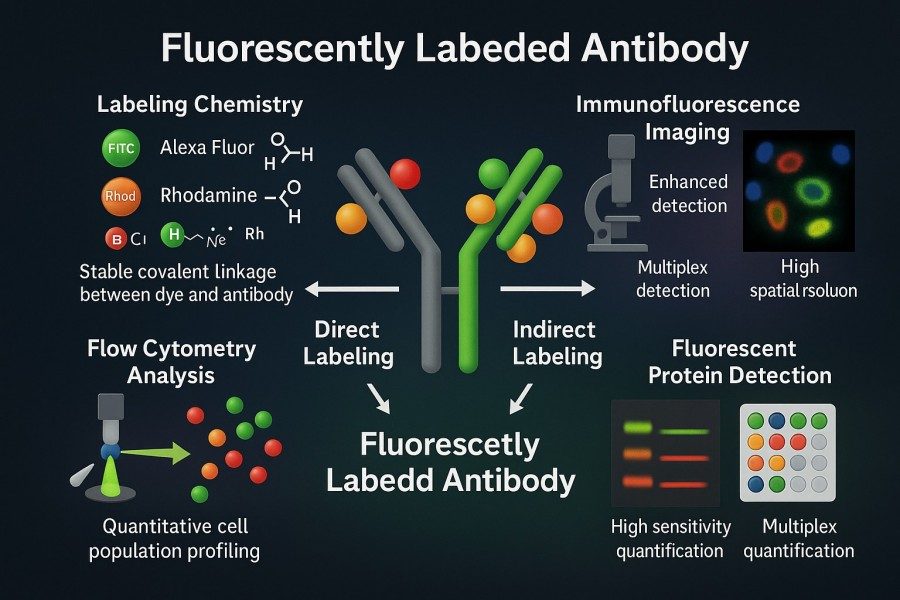 Fig. 1. Fluorescent antibodies (BOC Sciences Authorized).
Fig. 1. Fluorescent antibodies (BOC Sciences Authorized).
At the molecular level, the characteristics of fluorescent-labeled antibodies are determined by the antibody structure, the optical properties of the fluorophore, and the conjugation site between them. High-quality fluorescent antibodies require not only strong antibody affinity and batch-to-batch consistency but also bright, photostable fluorophores that minimally interfere with biological systems. Selecting the right antibody, fluorophore, and conjugation strategy is fundamental to establishing any fluorescence-based HTS method.
Advantages Over Traditional Detection Methods
Traditional protein detection methods, such as colorimetric assays (e.g., ELISA), chemiluminescence, and radioactive assays, have limitations in sensitivity, dynamic range, and automation compatibility. Fluorescent-labeled antibodies offer significant advantages, making them particularly suited for HTS:
- Higher sensitivity and wider dynamic range: Fluorescent antibodies provide bright signals with low background noise, enabling reliable detection at protein levels as low as picomolar, while covering a broad dynamic range suitable for quantitative analysis and dose-response studies.
- Stable, rapid, and real-time readout: Fluorescence detection does not require chromogenic or enzymatic reactions; signals can be read immediately, reducing well-to-well variation, accelerating experiments, and ensuring compatibility with automated systems.
- Multiplexing capabilities: Different fluorophores can detect multiple targets simultaneously, enabling multi-parameter analysis in a single well, which is especially useful in complex cell or pathway studies.
- Suitability for miniaturized formats: Fluorescence signals remain stable in 384- and 1536-well microplates, supporting high-density, high-throughput screening while reducing reagent consumption and experimental costs.
- Safer than radioactive or hazardous reagents: Fluorescent systems are safe, easy to handle, and readily adaptable to automation and large-scale applications, aligning with modern laboratory standards.
Why Fluorescent-Labeled Antibodies are Essential for HTS Systems?
HTS is a central approach in modern drug development, signaling pathway research, and phenotypic analysis, aiming to generate large amounts of reliable data with minimal time, high precision, and low cost. Fluorescent-labeled antibodies play an indispensable role in HTS. They provide specific signals, enable rapid quantification, and are highly compatible with automated platforms, miniaturized plates, and multiplexing strategies—meeting HTS requirements for throughput, sensitivity, reproducibility, and dynamic range. The following sections explore their importance in HTS across key dimensions.
Benefits for Automation and Miniaturized Assays
Automation and miniaturization are core trends in HTS. Fluorescent-labeled antibodies offer natural advantages in this context:
- Adaptation to small-volume assays: Modern HTS often uses 384-, 1536-, or higher-density microplates, with reaction volumes per well reduced to a few microliters or less. Fluorescent antibodies maintain sufficient signal strength in miniaturized formats, ensuring detection sensitivity and data reliability.
- Rapid readout and real-time monitoring: Fluorescence signals can be captured directly by plate readers or high-content imaging systems without chromogenic or radioactive processing, significantly reducing experimental time. For automated workflows, this enables continuous processing of thousands to tens of thousands of samples, enhancing screening efficiency.
- High compatibility with automated equipment: Fluorescent antibodies integrate seamlessly with liquid handling systems, robotic dispensers, automated incubation, and imaging platforms, reducing human error and ensuring well-to-well and batch-to-batch consistency.
- Reagent savings and cost reduction: Miniaturized formats reduce the amount of antibody and fluorophore required per well, balancing experimental costs with high-throughput data acquisition, particularly suitable for large-scale drug screening.
Enhancing Sensitivity and Dynamic Range
In HTS, detection sensitivity and dynamic range directly affect the ability to capture weak signals and large variations. Fluorescent antibodies offer notable advantages:
- High-sensitivity detection: High-quality fluorophores (e.g., Alexa Fluor, Cy series) have high quantum yields, providing clear signals even at picomolar protein concentrations. This is crucial for detecting low-expression targets, trace drug effects, or early signaling changes.
- Wide dynamic range: Fluorescence intensity can span several orders of magnitude, allowing researchers to detect both high- and low-expression proteins in the same screen, generating reliable dose-response curves and quantitative data.
- Signal tunability: Adjusting antibody concentration, incubation time, or fluorophore selection allows flexible optimization of signal strength, ensuring linearity and reproducibility.
- Compatibility with plates and imaging systems: Fluorescent antibodies maintain signal stability in 384-, 1536-well plates and high-content imaging systems, enabling high-sensitivity detection in parallel with high throughput.
Reducing Assay Variability and Background Signals
Consistency and low background are critical for HTS success. Fluorescent antibodies offer clear advantages in minimizing experimental variability and background:
- High specificity reduces non-specific binding: Fluorescent antibodies reduce off-target protein signal interference through highly specific antibody-antigen interactions, decreasing well-to-well and plate-to-plate variation.
- Stable fluorophores minimize signal drift: High-quality fluorophores are photostable, resisting photobleaching, ensuring consistent signals even during long incubations or multiple imaging cycles.
- Compatibility with diverse buffers: Fluorescent antibodies maintain stable signals across various buffers, blocking agents, and cell culture conditions, reducing errors from varying experimental conditions.
- Automation enhances consistency: Integration with robotic dispensing and automated plate reading further reduces human-induced variability, making large-scale HTS data more reliable.
- Lower background improves S/N ratio: Optimizing antibody selection, fluorophore combinations, and blocking strategies significantly enhances signal-to-noise ratio, reducing false positives and negatives, and improving reproducibility and confidence in results.
Principles of High-Throughput Screening Using Fluorescent Antibodies
The fundamental principles of using fluorescent-labeled antibodies in HTS involve experimental design, plate selection, labeling strategies, and factors that affect signal quality and reproducibility. Understanding these principles helps researchers establish robust, scalable, and high-throughput detection systems. Well-planned design not only improves data accuracy but also maximizes experimental throughput and information content.
Miniaturized Assay Formats (96-, 384-, 1536-Well Plates)
HTS trends toward miniaturization and automation, and plate format directly impacts throughput, sensitivity, and operational efficiency.
| Plate Format | Typical Volume/Well | Description / Use Case | Advantages | Limitations / Challenges |
|---|---|---|---|---|
| 96-Well Plates | 100–200 μL | Suitable for method development, antibody validation, or preliminary screening. | - Flexible handling- Simple equipment adaptation. | - Lower throughput. - Longer experimental time per run. |
| 384-Well Plates | 20–50 μL | Most commonly used modern HTS format. | - Balances throughput and sensitivity. - Suitable for medium-scale drug screens. - Supports automated dispensing, washing, and imaging, reducing human error. | - Less ideal for ultra-high-throughput applications. |
| 1536-Well Plates and Above | 5–10 μL | Suitable for ultra-high-throughput screening (uHTS). | - Significantly increases screening throughput. - Reduces reagent costs. | - Higher demands on antibody signal strength, fluorophore photostability, and automation precision. - Susceptible to edge effects and evaporation. |
Application Tips:
Fluorescent antibodies maintain a high signal-to-noise ratio even in miniaturized plates, ensuring reliable data. Plate format-specific adjustments in antibody concentration, incubation time, and detection instrument settings are necessary to match the experimental scale.
Direct vs. Indirect antibody Labeling Strategies in HTS
Fluorescent-labeled antibodies can be applied in HTS using either direct or indirect labeling strategies. Choosing the appropriate strategy affects experimental efficiency, signal intensity, and data reproducibility.
| Labeling Method | Description | Advantages | Disadvantages |
|---|---|---|---|
| Direct Labeling | Antibodies are covalently conjugated directly with fluorophores to form a single complex. | - Simplified workflow with fewer incubation steps, suitable for automation. - Stable signals with reduced non-specific interference. | - Limited signal amplification, which may be less effective for detecting low abundance proteins. |
| Indirect Labeling | Primary antibodies recognize the target protein, and secondary antibodies carrying fluorophores bind to the primary antibody, enabling signal amplification. | - Higher sensitivity, amplifying weak signals. - Suitable for low-abundance proteins or rare target detection. | - More complex workflow, requiring additional incubation and wash steps. - Increased well-to-well variability, less ideal for ultra-high-throughput automation. |
Application Advice:
In HTS, most screening experiments favor direct labeling to simplify operations and ensure data consistency. Indirect labeling is better suited for specific research scenarios requiring high sensitivity or signal amplification.
Key Factors Affecting Signal Quality and Reproducibility
The success of HTS depends on signal quality and data reproducibility. Major factors influencing fluorescent antibody detection include:
- Antibody specificity and affinity: High-affinity, highly specific antibodies provide reliable signals even at low target concentrations and reduce false positives.
- Fluorophore brightness and photostability: Bright, photostable fluorophores provide clear signals in microplate assays and maintain consistency during extended readings.
- Buffer systems and blocking strategies: Proper choice of buffers and blocking agents optimizes antibody binding efficiency, reduces non-specific adsorption, and improves the signal-to-noise ratio.
- Plate material and design: High-quality black-walled, clear-bottom plates minimize well-to-well light interference and ensure uniform reactions.
- Automation precision: Accurate automated dispensing reduces well-to-well variation and ensures HTS data reproducibility.
- Temperature control and incubation conditions: Stable temperature and incubation help maintain cell state and fluorescence signal consistency, reducing batch-to-batch variation.
Selecting the Right Fluorophores for HTS Applications
In HTS, the performance of fluorescent antibodies largely depends on the chosen fluorophore. Different fluorophores vary in brightness, photostability, spectral properties, and multiplexing capability. Selecting the right fluorophore enhances detection sensitivity, dynamic range, and signal-to-noise ratio, while minimizing well-to-well and plate-to-plate variability, improving the reliability of multi-parameter experiments.
Brightness, Photostability, and Compatibility with Plate Readers
Fluorophore brightness, photostability, and compatibility with detection instruments are critical factors affecting HTS signal quality.
| Metric | Description |
|---|---|
| Brightness | Determined by the fluorophore's quantum yield and extinction coefficient. High-brightness fluorophores provide detectable signals even at low protein expression levels or in small-volume wells, improving experimental sensitivity. Common high-brightness fluorophores include Alexa Fluor series, DyLight series, and Cy series. |
| Photostability | Photobleaching reduces signal over time, affecting continuous readings and multiple imaging cycles. Highly photostable fluorophores are especially important in HTS, particularly for high-content imaging (HCI) or long-duration drug treatment experiments. Common photostable fluorophores include Alexa Fluor 488/555/647 and Atto series. |
| Compatibility with Plate Readers | Fluorophore excitation/emission wavelengths must match the optical filters of plate readers or imaging systems. Poor compatibility lowers the signal-to-noise ratio and may produce false signals. Selecting fluorophores based on instrument optics during experimental design is recommended to avoid ineffective measurements. |
Reducing Crosstalk and Spectral Overlap
In multiplexed assays, crosstalk and spectral overlap are common issues that can lead to false positives, signal distortion, and irreproducible data.
| Metric | Description |
|---|---|
| Fluorophore selection strategy | Choose fluorophores with large excitation/emission wavelength separation and narrow spectral peaks (e.g., FITC vs. Cy5, Alexa Fluor 488 vs. Alexa Fluor 647). |
| Optical filter optimization | Use narrow-band filters to limit detection wavelengths and reduce adjacent channel interference. Software compensation can correct spectral overlap in multi-channel imaging. |
| Signal correction and standardization | Use single-stain controls to calculate crosstalk coefficients. Data analysis can then compensate signals, ensuring accuracy in multi-target detection. |
| Experimental design considerations | Avoid combining fluorophores with high spectral overlap. For targets with large intensity differences, consider adjusting antibody concentrations or using signal amplification strategies. |
Common Fluorophores Used for Antibody Labeling
| Catalog | Name | CAS | Color | Ex (nm) | Em (nm) | Applications | Inquiry |
|---|---|---|---|---|---|---|---|
| A19-0041 | Hoechst 33258 | 23491-45-4 | Blue | ~352 | ~454 – 463 | Nuclear/DNA staining in live or fixed cells; cell cycle assays; fluorescence microscopy; flow cytometry (FACS). | Bulk Inquiry |
| F02-0110 | DyLight 3 CEP | 182873-76-3 | Violet–Blue | ~350–405 | ~420–460 | Likely nucleic acid or protein labeling — nuclear/DNA staining or cell imaging (assuming similar to Hoechst/UV–blue dyes). | Bulk Inquiry |
| R01-0042 | AF594 activated ester, 5-isomer | 1638544-48-5 | Red | ~590 | ~617 | Conjugation to proteins/antibodies/peptides/ amine-modified oligos; immunofluorescence microscopy; flow cytometry; multicolor labeling. | Bulk Inquiry |
| R01-0471 | AF647 NHS ester | 407627-60-5 | Far-red | ~650 | ~668 | Protein/antibody labeling; immunofluorescence; flow cytometry; deep-tissue or multiplex far-red imaging. | Bulk Inquiry |
| R01-0451 | AF 488 TFP ester | 2133404-55-2 | Green | ~495 | ~519 | Protein/antibody/peptide labeling; immunofluorescence; flow cytometry; general green-channel fluorescence microscopy. | Bulk Inquiry |
| R01-0044 | AF488 azide | 1679326-36-3 | Green | ~495 | ~519 | Bio-orthogonal labeling (click chemistry) of biomolecules; immunofluorescence; flow cytometry; general fluorescence microscopy. | Bulk Inquiry |
| R01-0022 | Cyanine7 NHS ester | 1432019-64-1 | Near-IR / far-red | ~750 | ~ 779 | Conjugation to proteins/antibodies; in-vivo imaging; microarrays; NIR fluorescence imaging; multiplex assays. | Bulk Inquiry |
| F02-0016 | Cyanine7 carboxylic acid | 1628790-40-8 | Near-IR | ~740 | ~770 | As fluorescent tag standard (non-reactive); reference dye; may be converted to reactive form for labeling; NIR imaging, in-vivo or in-vitro. | Bulk Inquiry |
| F02-0014 | Cyanine7 amine | 1650635-41-8 | Near-IR | ~750 | ~776 | Protein/peptide labeling via amine coupling; antibody conjugation; NIR imaging; microarrays. | Bulk Inquiry |
| F02-0015 | Cyanine7 azide | 1557397-59-7 | Near-IR | ~750 | ~776 | Bio-orthogonal labeling (click chemistry); conjugation to alkyne-modified biomolecules; NIR imaging; multiplex fluorescence assays. | Bulk Inquiry |
Note: Please verify with the vendor's technical datasheet or MSDS before actual use.
Looking for Antibody Labeling Dyes?
We provide flexible conjugation options with various fluorophores, including water-soluble and photostable dyes, to meet your experimental requirements.
Key Applications of Fluorescent Antibodies in HTS
Fluorescent-labeled antibodies play a central role in HTS due to their high sensitivity and high-resolution detection capabilities. They enable dynamic single-cell analysis and simultaneous monitoring of multiple biomolecules, allowing complex pathway and multi-marker analysis. These features make fluorescent antibodies widely applied in drug screening, protein function studies, and disease mechanism research, providing researchers with reliable, quantitative data.
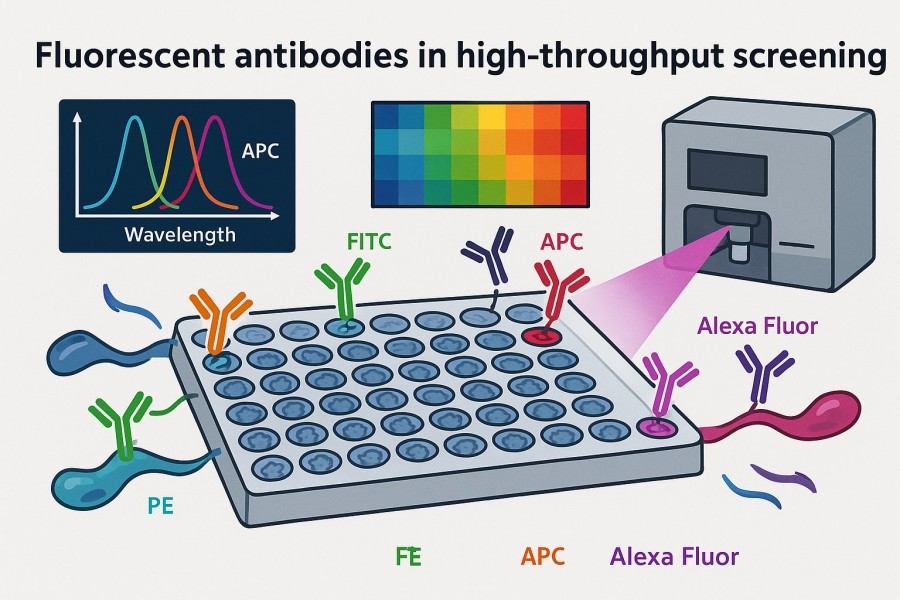 Fig. 2. Fluorescent antibodies in high-throughput screening (BOC Sciences Authorized).
Fig. 2. Fluorescent antibodies in high-throughput screening (BOC Sciences Authorized).
High-Content Imaging–Based Screening
High-Content Screening (HCS) combined with fluorescent antibody technology captures complex intracellular dynamics. Through multi-channel imaging and advanced image analysis, HCS simultaneously evaluates protein distribution, pathway activation, and cellular morphology, providing detailed data for drug mechanism studies and phenotypic screening. This approach is ideal for high-resolution, multi-parameter research. Common applications include:
- Subcellular localization changes: Track protein translocation within the nucleus, mitochondria, or membranes under different treatment conditions.
- Pathway activation: Monitor the activation state of signaling molecules, such as transcription factors or phosphorylated proteins, revealing drug or stimulus effects.
- Protein transcription/translation dynamics: Observe protein expression changes over time and quantify transcription and translation levels for drug effect assessment.
- Cell morphology changes: Analyze changes in cell shape, cytoskeleton, and membrane structures to assess stress responses, apoptosis, or migration.
Multiplexed Biomarker Detection
Multiplexed fluorescent antibody technology allows simultaneous detection of multiple biomarkers in a single well, greatly increasing experimental efficiency and data density. It enables complex signaling network analysis and disease marker screening from limited samples, allowing researchers to evaluate the functional state of drug candidates or biological specimens more comprehensively. Common applications include:
- Immune signaling network analysis: Detect multiple signaling molecules and their phosphorylation states simultaneously to reveal dynamic pathway networks.
- Tumor-related biomarker screening: Quantitatively analyze multiple cancer-related proteins in a single experiment to quickly identify potential therapeutic targets.
- Protein phosphorylation detection: Measure phosphorylation modifications of various proteins to understand cellular responses to stimuli or drugs.
- Improving data density and experimental efficiency: Multi-marker detection reduces reagent and sample usage while increasing the amount of biological information obtained.
Cell-Based Functional Assays and Pathway Profiling
Cell-based functional assays are critical in drug development and mechanism studies. Fluorescent antibody technology allows real-time monitoring of receptor activation, signaling pathway regulation, and cellular physiological changes in vitro. Dynamic evaluation of cell function helps researchers quickly identify mechanisms of action for drug candidates and provides data for subsequent optimization. Common applications include:
- GPCR activation analysis: Monitor G-protein-coupled receptor activity and downstream effectors to assess drug impact on receptor signaling.
- Kinase pathway activity monitoring: Track pathway activation or inhibition by fluorescently labeling key kinases or substrate proteins.
- Apoptosis and cell cycle screening: Assess cell death, proliferation, or cycle changes to evaluate drug-induced cellular responses.
- Immune cell activation studies: Monitor activation, migration, and secretion functions of T cells, B cells, and other immune cells.
Optimizing Fluorescent Antibody Performance in HTS Workflows
Optimizing fluorescent antibody performance is essential for obtaining reliable, high-quality data in HTS. This includes fluorophore selection, antibody design, experimental condition optimization, and adjustment of automated equipment parameters. Proper optimization at each step not only improves signal specificity and stability but also ensures data reproducibility and comparability.
Selecting the Right Fluorophore for Assay Compatibility
Choosing the appropriate fluorophore is critical to the success of HTS. Fluorophores must match the antibody's binding characteristics, be compatible with detection instruments, and remain stable in assay buffer systems. In addition, plate material and background fluorescence can impact signal quality, so selection should consider spectral properties, stability, and instrument compatibility.
- Antibody-target binding kinetics: Different antibodies have varying affinities and binding rates, directly affecting signal intensity and uniformity.
- Fluorophore spectral compatibility with instruments: Select fluorophores whose excitation/emission wavelengths match plate readers, flow cytometers, or high-content imaging systems to ensure signals are fully captured without instrument limitations.
- Effect of assay buffers on fluorophore stability: Some buffers or additives may cause photobleaching or reduced emission efficiency, so compatibility should be verified in advance.
- Plate background interference: Different microplate types (black-walled, clear-bottom, or white-walled) reflect or absorb fluorescence differently. Choosing the appropriate plate reduces background noise and improves signal-to-noise ratio.
Minimizing Spectral Overlap and Crosstalk
In multiplexed fluorescence assays, spectral overlap and crosstalk between channels can affect quantitative accuracy. By selecting well-separated fluorophores, using narrow-band filters, multi-exposure strategies, and mathematical compensation, crosstalk can be significantly reduced, enhancing multi-channel detection reliability and precision. Approaches include:
- Using narrow-band filters: Precisely filter non-target wavelengths to reduce interference between fluorophores.
- Selecting dyes with well-separated emission peaks: In multiplexed experiments, choose fluorophore combinations with large spectral differences.
- Implementing multi-exposure strategies: Optimize exposure times for each channel during imaging or plate reading to prevent strong signals from masking weaker ones.
- Mathematical compensation and spectral unmixing: Use instrument software to deconvolute or linearly compensate signals, correcting minor spectral overlaps to ensure accurate quantification.
Improving Signal-to-Noise Ratio for Reliable Readouts
A high signal-to-noise ratio (S/N) is fundamental for reliable HTS data. S/N is influenced by antibody concentration, incubation time, background interference, and imaging conditions. Optimizing antibody parameters, selecting low-background materials, and controlling illumination can effectively enhance data quality. Strategies include:
- Optimizing antibody concentration and incubation time: Excessive or insufficient antibody can reduce specific signals or increase background; adjust according to target abundance.
- Using blocking buffers to reduce non-specific binding: Appropriate blockers (e.g., BSA, milk protein, or commercial buffers) significantly reduce non-specific adsorption, improving S/N.
- Using black-walled plates to minimize stray light: Particularly in fluorescence imaging and plate reading, black-walled plates suppress inter-well crosstalk, enhancing detection accuracy.
- Controlling temperature and light exposure: Fluorescent signals are prone to photobleaching; managing experimental temperature and minimizing prolonged light exposure maintains signal stability.
Considerations for Assay Scaling and Automation
In automated and large-scale HTS, experimental consistency and reproducibility are critical. Liquid handling precision, pipetting accuracy, well-to-well evaporation, and batch timing differences can all affect results. Proper experimental workflow design and automated system parameter control minimize errors and ensure reliable data. Key considerations include:
- Pipetting consistency (CV values): Liquid handling precision directly affects well-to-well signal uniformity; regularly calibrate pipetting systems.
- Residual volume in tips: Residual liquid may cause cross-contamination or signal bias; use low-retention tips or optimize washing steps.
- Well-to-well evaporation: During long-term or high-temperature experiments, edge wells may evaporate, causing concentration deviations; use plate seals or humidity control.
- Consistency in batch timing: In large-scale HTS, operations across different batches should be synchronized to avoid systematic errors and maintain data reproducibility.
Common Challenges in High-Throughput Fluorescence Assays
Although fluorescent antibodies provide sensitive, fast, and high-resolution detection signals in HTS, practical applications still face technical challenges. These issues can impact signal stability, data consistency, and experimental reproducibility, limiting the reliability of screening results. Understanding these challenges and their causes, and applying targeted optimization strategies, is key to establishing a robust, efficient HTS platform.
Managing Photobleaching and Fluorescence Decay
Signal decay over time (photobleaching) is common in HTS, especially during long-term imaging or high-intensity laser exposure. Photobleaching reduces signal strength and affects quantitative analysis accuracy. Effective strategies include:
- Selecting more photostable fluorophores: Use photobleaching-resistant dyes or modified fluorescent antibodies to extend signal duration.
- Reducing exposure time and laser intensity: Optimize imaging parameters to use minimal excitation energy while obtaining detectable signals, minimizing photodamage.
- Adding anti-quenching agents: Include agents like Trolox or reactive oxygen scavengers in buffers to slow fluorescence decay.
- Implementing rapid imaging strategies: Use fast scanning or multi-point acquisition to reduce per-sample light exposure and improve data consistency.
Avoiding Non-Specific Binding
Non-specific binding increases background signals, lowering S/N and affecting accurate target detection. Mitigation strategies include:
- Using appropriate blocking agents: BSA, non-fat dry milk (NFDM), or specialized blocking buffers occupy non-specific sites and reduce background.
- Optimizing antibody concentration: Excessive antibody may increase non-specific binding, while too little reduces signal; titration experiments help determine optimal working concentrations.
- Enhancing washing steps: Increase wash cycles or optimize wash buffer conditions to remove unbound or weakly bound antibodies.
- Selecting high-affinity antibodies: Highly specific antibodies better distinguish target from non-specific binding, reducing background.
Troubleshooting Data Inconsistency Across Plates
In multi-plate HTS experiments, data consistency is often affected by multiple factors, leading to significant variation between plates or batches. Common issues and solutions include:
- Edge effects: Edge wells are prone to evaporation and temperature variation; fill edge wells with buffer or compensate in plate design.
- Temperature gradients affecting cell states: Control environmental temperature and humidity to ensure uniform conditions across all plates.
- Liquid handling errors in automation systems: Regularly calibrate pipettes and dispensing devices to reduce uneven distribution or volume errors.
- Uneven fluorophore distribution: Optimize antibody dispensing and mixing to ensure uniform fluorescent labeling across samples.
Comprehensive Fluorescent Antibody Solutions by BOC Sciences
BOC Sciences is committed to providing high-quality, reliable fluorescent antibody solutions for research and drug development. With years of expertise and advanced technology platforms, we support the entire workflow from fluorophore production to high-throughput screening, helping clients achieve precise and reproducible experimental outcomes.
Fluorophore Production and Modification Services
- Synthesize common and custom fluorophores, including FITC, Rhodamine, and Cy series, ensuring high purity and stability.
- Support chemical modifications, such as NHS esterification, maleimide functionalization, and hydrophilic modifications, to improve labeling efficiency and antibody compatibility.
- Optimize fluorophore spectral properties per customer requirements for perfect compatibility with instruments or multicolor imaging systems.
- Provide quality control and analytical reports for small-molecule fluorophores before conjugation to large biomolecules, ensuring batch consistency.
Fluorescent Antibody Conjugation Services
- Efficiently covalently conjugate monoclonal or polyclonal antibodies while maintaining fluorophore and antibody activity.
- Offer multiple conjugation strategies (amine, thiol, etc.) to suit different antibody types and experimental designs.
- Optimize antibody-to-fluorophore ratios and incubation conditions for high specificity and strong signal.
- Support single- or multi-color labeling for complex assays and multiplexed applications.
One-Stop High-Throughput Screening Fluorescent Solutions
- Provide customized fluorescent antibody solutions for HTS, including antibody selection, fluorophore matching, and automation compatibility optimization.
- Offer guidance on plate design, dispensing strategies, and signal optimization to improve screening efficiency and data reproducibility.
- Support multi-channel detection, multicolor imaging, and high-content screening workflows.
- Assist clients in reducing background signals, minimizing spectral crosstalk, and enhancing S/N in HTS processes.
Full Analytical Support for Quality Control
- Provide comprehensive analyses before and after fluorescent antibody conjugation, including SDS-PAGE, HPLC, mass spectrometry, and fluorescence spectroscopy validation.
- Quantitatively assess antibody activity, degree of labeling (DOL), and signal intensity to ensure experimental reliability.
- Supply batch records, quality reports, and stability data to meet rigorous research and clinical requirements.
- Support custom validation needs, such as multicolor flow cytometry, immunofluorescence imaging, or HTS data consistency verification.
Do You Need A Consultation?
BOC Sciences integrates cutting-edge fluorescence technologies to accelerate your research, driving next-generation solutions for drug discovery and diagnostics.
References
- Vira S, et al. Fluorescent-labeled antibodies: Balancing functionality and degree of labeling. Anal Biochem. 2010; 402(2):146–150. DOI: 10.1016/j.ab.2010.03.036. PMID: 20362543.
- Fang X, et al. Recent Advances in Design of Fluorescence-Based Assays for High-Throughput Screening. Anal Chem. 2019; 91(1):482–504. DOI: 10.1021/acs.analchem.8b05303. PMID: 30481456.
- Wang XD, et al. High-throughput strategies for monoclonal antibody screening: advances and challenges. J Biol Eng. 2025; 19(1):41. DOI: 10.1186/s13036-025-00513-z. PMID: 40340930.
- Riedl T, et al. High-Throughput Screening for Internalizing Antibodies by Homogeneous Fluorescence Imaging of a pH-Activated Probe. J Biomol Screen. 2016; 21(1):12–23. DOI: 10.1177/1087057115613270. PMID: 26518032.
Transform Your Studies with Cutting-Edge Fluorescent Products
| Catalog | Name | CAS | Inquiry |
|---|---|---|---|
| A19-0040 | Hoechst 33342 | 23491-52-3 | Bulk Inquiry |
| F04-0012 | FITC isomer I | 3326-32-7 | Bulk Inquiry |
| F02-0026 | Cy5-NHS ester | 146368-14-1 | Bulk Inquiry |
| A16-0201 | DAPI dihydrochloride | 28718-90-3 | Bulk Inquiry |
| F02-0112 | DyLight 5 CEP | 182873-67-2 | Bulk Inquiry |
| F02-0030 | Cy3-NHS ester | 146368-16-3 | Bulk Inquiry |
| A19-0060 | Hoechst 34580 | 23555-00-2 | Bulk Inquiry |
| A19-0034 | DAPI dilactate | 28718-91-4 | Bulk Inquiry |
| R01-0472 | Atto 425-NHS ester | 892156-28-4 | Bulk Inquiry |
| A16-0019 | Tetramethylrhodamine isothiocyanate (mixed isomers) | 95197-95-8 | Bulk Inquiry |
High-Performance Fluorescent Tools for Your Research
- TAMRA Dyes Red-emitting dyes for antibody and protein labeling.
- ATTO Dyes High-performance dyes for labeling and imaging experiments.
- Coumarin Blue-emitting dyes for chemical sensing and fluorescence studies.
- Fluorescent Protein Used for live-cell imaging and real-time biosensing.
More About Antibody labeling
Online Inquiry

