Flow Cytometry – 8 Common Sample Preparation and Intracellular Staining Protocols
01-27-2026
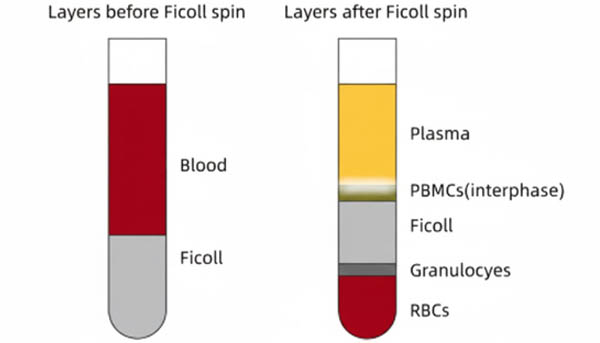
Flow cytometry (FCM), as a precise technology for multi-parameter quantitative analysis of heterogeneous cell populations at the single-cell level, relies heavily on the quality of sample pre-processing for accurate and reliable data. Preparing highly viable, non-aggregated single-cell suspensions with stable antigen expression is the cornerstone for subsequent fluorescence compensation, population gating, and signal analysis. Maintaining cell viability, removing dead cell debris, and excluding doublets directly affect the signal-to-noise ratio (S/N ratio) and the effectiveness of gating strategies. Physical dissociation tailored to tissue mechanics, such as spleen, bone marrow, or thymus, or density gradient centrifugation and red blood cell lysis for peripheral blood, must strictly follow standardized operating procedures (SOPs) to prevent excessive cell damage or enzymatic/epitope loss. For intracellular targets like cytokines and transcription factors, precise fixation and permeabilization are crucial for crossing biological membranes, ensuring antibody access, and maintaining specific binding. This article systematically outlines eight typical single-cell preparation and intracellular staining protocols, providing standardized references for establishing high-quality flow cytometry workflows.
Flow Cytometry – Antibody Staining Protocol (Surface and Intracellular)
01-27-2026
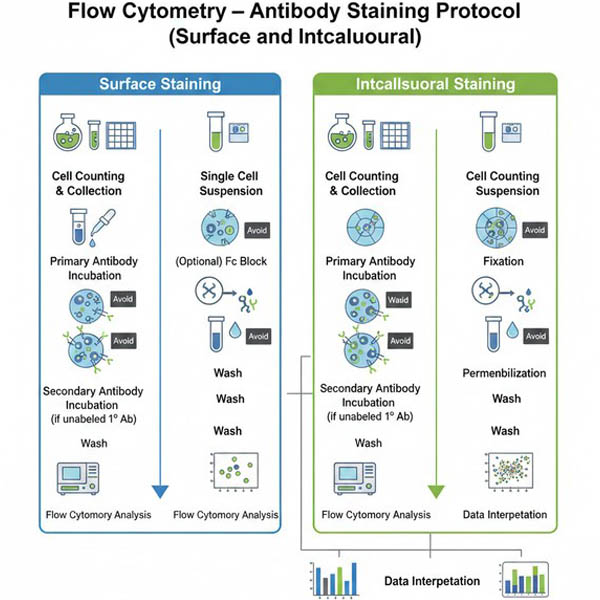
Flow cytometry antibodies are designed for use in flow cytometry experiments. Compared to antibodies used in ELISA, Western blotting (WB), or immunofluorescence (IF), they generally require less complex sample preparation and experimental steps. Flow cytometry antibodies can detect either live or fixed cells and provide quantitative, single-cell, multi-parameter data. They are available as either unlabeled or fluorescently labeled antibodies. Unlabeled antibodies require a corresponding secondary antibody, while fluorescently labeled antibodies carry the fluorophore and do not require a secondary antibody.
Flow Cytometry – Intracellular and Nuclear Factor Staining Protocol
01-27-2026
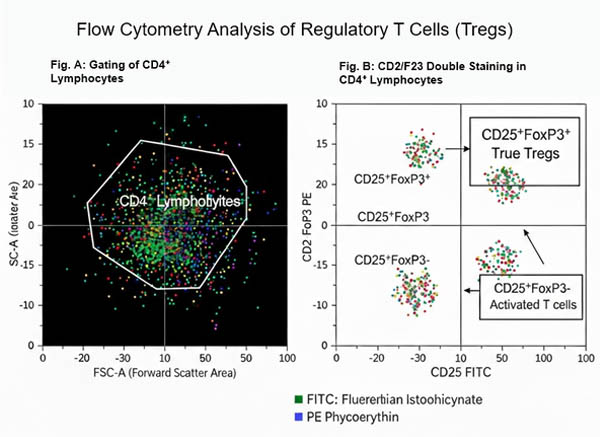
Staining and detecting intracellular molecules helps identify cell subpopulations and states. Unlike traditional surface antibody staining, flow cytometry analysis of intracellular proteins requires initial fixation to stabilize the cell membrane, followed by permeabilization to allow antibodies to access intracellular antigens. Mass cytometry extends this capability by simultaneously analyzing both surface markers and intracellular or nuclear components across multiple channels, without the need for voltage adjustment or compensation, greatly enhancing detection power.
Flow Cytometry - Surface and Intracellular Cell Staining Protocol
01-27-2026
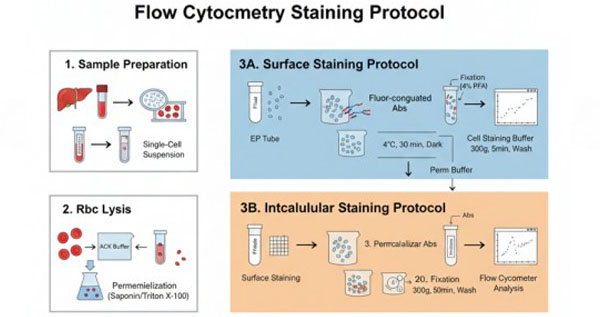
Flow cytometry (FCM) is a single-cell–based quantitative analysis and sorting technology performed using a flow cytometer. Flow cytometry is a high-technology platform developed through the advanced integration of monoclonal antibody and immunocytochemistry techniques, laser technology, and computer science. It enables effective discrimination of heterogeneous cell populations at the single-cell level. Detection targets include, but are not limited to, suspended cells, adherent cells, single-cell suspensions dissociated from solid tissues, and other biological particles.

