Flow Cytometry – Antibody Staining Protocol (Surface and Intracellular)
Flow cytometry antibodies are designed for use in flow cytometry experiments. Compared to antibodies used in ELISA, Western blotting (WB), or immunofluorescence (IF), they generally require less complex sample preparation and experimental steps. Flow cytometry antibodies can detect either live or fixed cells and provide quantitative, single-cell, multi-parameter data. They are available as either unlabeled or fluorescently labeled antibodies. Unlabeled antibodies require a corresponding secondary antibody, while fluorescently labeled antibodies carry the fluorophore and do not require a secondary antibody.
Flow Cytometry Antibody Staining Protocols
Cell Surface Staining Steps
- Cell counting: Collect cultured cells into a 15 mL centrifuge tube and count them. For adherent cells, digest with trypsin first, centrifuge at 300 × g for 5 min, and discard the supernatant.
- Prepare a single-cell suspension: Resuspend collected cells in 1 mL of pre-chilled 0.5% BSA/PBS, centrifuge at 300 × g for 5 min, discard the supernatant, and repeat twice. Resuspend cells in 0.5% BSA/PBS at a concentration of 1 × 10⁷ cells/mL, and transfer 100 μL into a flow cytometry tube.
- (Optional) Fc receptor blocking: Mix cells with species-matched whole IgG antibody and incubate at room temperature for 10–20 min.
- Primary antibody incubation: Add 100 μL of diluted primary antibody per tube, incubate at 2–8°C for 30 min, gently flick the tube every 10 min to ensure thorough mixing. For directly conjugated antibodies, protect from light and skip to step 7. Antibody diluent: 0.5% BSA/PBS.
- Washing: Centrifuge at 300 × g for 5 min, discard supernatant, resuspend in 200 μL 0.5% BSA/PBS, centrifuge again, and repeat twice.
- Secondary antibody incubation: For non-fluorescently labeled primary antibodies, add 100 μL fluorescent secondary antibody, incubate at 2–8°C for 30 min in the dark, flicking every 10 min.
- Washing: Repeat washing step as in step 5.
- Analysis: Resuspend cells in 200 μL 0.5% BSA/PBS, store at 4°C, and proceed to flow cytometry analysis.
Intracellular Staining Steps
- Cell counting: Collect cultured cells into a 15 mL centrifuge tube and count. For adherent cells, digest with trypsin, centrifuge at 300 × g for 5 min, and discard supernatant.
- Prepare a single-cell suspension: Resuspend cells in 1 mL 0.5% BSA/PBS, centrifuge at 300 × g for 5 min, discard supernatant, and repeat twice. Resuspend at 1 × 10⁷ cells/mL, transfer 100 μL to a flow cytometry tube, centrifuge at 300 × g for 5 min, discard supernatant.
- Fixation: Add 100 μL of cell fixation solution, incubate at 2–8°C for 20 min. (e.g., 4% paraformaldehyde)
- Washing: Centrifuge at 600 × g for 5 min, discard supernatant, resuspend in 200 μL 0.5% BSA/PBS, centrifuge and repeat twice.
- Permeabilization: Add 100 μL permeabilization solution, incubate at room temperature for 20 min. (e.g., 0.5% BSA/PBS + 0.3% Triton X-100)
- Washing: Repeat washing step as above.
- (Optional) Fc receptor blocking: Mix cells with species-matched whole IgG antibody, incubate at room temperature for 10–20 min.
- Primary antibody incubation: Add 100 μL of diluted primary antibody per tube, incubate at 2–8°C for 30 min, gently flick every 10 min. For directly conjugated antibodies, protect from light and skip to step 11. Antibody diluent: 0.5% BSA/PBS.
- Washing: Centrifuge at 600 × g for 5 min, discard supernatant, resuspend in 200 μL 0.5% BSA/PBS, centrifuge and repeat twice.
- Secondary antibody incubation: For non-fluorescent primary antibodies, add 100 μL fluorescent secondary antibody, incubate at 2–8°C for 30 min in the dark, flicking every 10 min. For directly conjugated antibodies, skip to step 11.
- Washing: Repeat washing step as above.
- Analysis: Resuspend cells in 200 μL 0.5% BSA/PBS, store at 4°C, and proceed to flow cytometry analysis.
Note: Include an unstained control and isotype control. The unstained control receives no primary antibody, while the isotype control uses a non-specific antibody in place of the primary antibody.
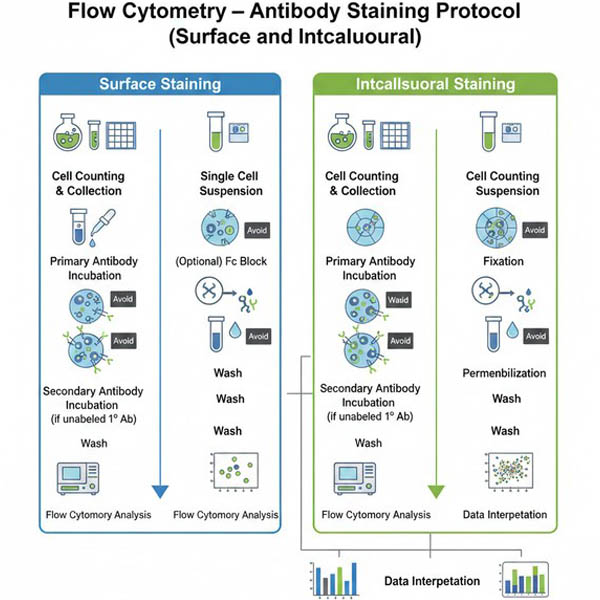 Fig. 1. Comprehensive workflow comparing key steps for cell surface and intracellular staining (BOC Sciences Authorized).
Fig. 1. Comprehensive workflow comparing key steps for cell surface and intracellular staining (BOC Sciences Authorized).
Guide for Flow Cytometry: Optimization, Controls, and Troubleshooting
High-quality flow cytometry results depend not only on standardized protocols but also on rigorous experimental design and scientific quality control. Beyond basic surface or intracellular staining, researchers face practical challenges such as excluding dead cells, adjusting multi-color compensation, and optimizing antibody concentrations.
Live/Dead Discrimination
Before surface or intracellular staining, it is recommended to perform a viability stain to exclude dead cells.
- Non-fixed samples (surface staining): Add PI (Propidium Iodide) or 7-AAD immediately before analysis.
- Recommended concentration: PI 1–5 μg/mL, 7-AAD 5–10 μg/mL.
- Incubation: 5–10 min at room temperature in the dark; no washing required, proceed directly to analysis.
- Fixed samples (intracellular staining): Use fixable viability dyes; PI/7-AAD are not suitable as they will be removed or give false-positive signals after fixation/permeabilization.
- Perform viability staining before surface staining.
- Resuspend cells in protein-free PBS, incubate with dye in the dark for 15–30 min.
- Wash before proceeding with surface staining and fixation/permeabilization steps.
Essential Controls
To ensure data accuracy, in addition to unstained and isotype controls, multi-color experiments should include:
- Single-Stained Controls/Compensation Controls:
- Purpose: Adjust fluorescence compensation and correct spectral overlap.
- Sample: Cells or compensation beads (recommended if cell number is limited or antigen expression is low).
- Fluorescence Minus One (FMO) Controls:
- Purpose: Accurate gating in multi-color panels, more precise than isotype controls for defining negative/positive populations.
- Procedure: Include all antibodies except the one of interest.
Common Buffer Recipes
Staining Buffer:
- Composition: 1× PBS (pH 7.4) + 0.5%–2% BSA (or 2–5% FBS) + 0.09% NaN₃ (optional preservative).
- Note: When using a biotin–streptavidin system, do not use skim milk containing endogenous biotin for blocking.
Fixation Buffer:
- Composition: 1–4% paraformaldehyde (PFA) in PBS, pH 7.4. Prepare fresh or store aliquots at −20°C.
Permeabilization Buffer:
- Cytoplasmic proteins: Staining Buffer + 0.1–0.5% saponin (reversible; include in wash buffer) or 0.1% Triton X-100.
- Nuclear proteins / transcription factors: It is recommended to use specialized commercial nuclear permeabilization kits for optimal results.
Troubleshooting Guide
| Observation | Possible Cause | Solution |
|---|---|---|
| No/weak signal | Antibody concentration too low or low affinity | Optimize by titration; ensure correct secondary antibody |
| Fluorescence quenching | Protect from light; check cytometer lasers | |
| Intracellular antigen not exposed | Optimize fixation/permeabilization | |
| High background/non-specific staining | Insufficient blocking | Increase Fc receptor blocking; raise BSA concentration |
| Antibody too concentrated | Centrifuge to remove aggregates; dilute antibody | |
| Dead cell interference | Use viability dye to exclude dead cells | |
| Inadequate washing | Increase wash steps or volume | |
| Poor population separation | Improper compensation | Recalculate using single-stained controls |
| Severe spectral overlap | Redesign panel to avoid overlapping fluorophores |
Fluorescent Dyes Recommended for Your Research Project
| Catalog | Name | CAS | Inquiry |
|---|---|---|---|
| R10-0005 | 6-Fluorescein phosphoramidite | 204697-37-0 | Bulk Inquiry |
| F04-0033 | 5-Aminofluorescein | 3326-34-9 | Bulk Inquiry |
| F04-0012 | FITC isomer I | 3326-32-7 | Bulk Inquiry |
| A16-0201 | DAPI dihydrochloride | 28718-90-3 | Bulk Inquiry |
| F04-0034 | 5-Carboxyfluorescein diacetate | 79955-27-4 | Bulk Inquiry |
| F04-0055 | Dexamethasone Fluorescein | 216854-76-1 | Bulk Inquiry |
| A16-0033 | 6-Carboxyfluorescein | 3301-79-9 | Bulk Inquiry |
| A16-0004 | Phalloidin-FITC | 915026-99-2 | Bulk Inquiry |
| A19-0034 | DAPI dilactate | 28718-91-4 | Bulk Inquiry |
| A17-0083 | Cy7-NHS ester | 477908-53-5 | Bulk Inquiry |
| A17-0061 | Rhodamine 610 Perchlorate | 23857-51-4 | Bulk Inquiry |
| A17-0107 | Rhodamine 640 Perchlorate | 72102-91-1 | Bulk Inquiry |
| A17-0181 | N-(m-PEG4)-N'-(azide-PEG4)-Cy7 | 2107273-40-3 | Bulk Inquiry |
| F04-0032 | FAM isothiocyanate (FITC), 5- and 6-isomers | 27072-45-3 | Bulk Inquiry |
| A17-0178 | Cy5.5 bis-NHS ester | 2183440-77-7 | Bulk Inquiry |
| F02-0099 | Cy5.5-NHS ester tetrafluoroborate | 2375105-86-3 | Bulk Inquiry |
| R12-0004 | Texas Red-X | 199745-67-0 | Bulk Inquiry |
| R01-0471 | AF647 NHS ester | 407627-60-5 | Bulk Inquiry |
Corporate Profile
BOC Sciences serves global research and industrial customers, focusing on the development, production, and supply of fluorescent products and labeling services. Leveraging a mature chemical synthesis and functional materials platform, the company has built a comprehensive product portfolio covering small-molecule fluorescent dyes, fluorescently labeled antibodies, in vivo imaging probes, and molecular imaging tools, which are widely applied in flow cytometry, immunology research, molecular mechanism studies, drug screening, and advanced bioimaging.
The company offers a diverse range of fluorescent labeling and customization services, including antibody conjugation, spectral tuning of fluorophores, dye structure optimization, and experimental protocol support, meeting the needs of researchers for surface and intracellular factor staining, multicolor flow cytometry, and live-cell analysis.
BOC Sciences is supported by a research and development team of experienced chemists and biologists, equipped with advanced synthesis, purification, and analytical characterization instruments, and has established a strict quality control system to ensure the reliability of each fluorescent product in terms of purity, stability, and optical performance. Our fluorescent probes and labeling services have been utilized by universities, research institutions, and biopharmaceutical companies worldwide for cell imaging, molecular tracing, mechanism studies, and method development, and have been validated and cited in numerous scientific publications. We are committed to being your trusted long-term partner in fluorescent labeling and imaging research.
High-Performance Fluorescent Tools for Your Research
- BODIPYBright, stable dyes used in bioimaging and molecular labeling.
- CoumarinBlue-emitting dyes for chemical sensing and fluorescence studies.
- AF DyesBright, versatile dyes for bioimaging applications.
- Fluorescein FAMGreen fluorescent dye for nucleic acid labeling.
- CyanineVersatile dyes used in biosensing and nucleic acid detection.
- TAMRA DyesRed-emitting dyes for antibody and protein labeling.
- ATTO DyesHigh-performance dyes for labeling and imaging experiments.
- Fluorescent ProteinUsed for live-cell imaging and real-time biosensing.
Custom Fluorescent Solutions Designed for Your Experiments
- DNA StainingPrecise fluorescent dyes for clear DNA visualization and analysis.
- Lipid StainingFluorescent solutions for effective lipid structure imaging.
- Cell StainingAdvanced staining for detailed cell morphology and analysis.
- Protein StainingHigh-quality staining for accurate protein detection and imaging.
- Bacteria ImagingFluorescent solutions to visualize bacterial structures and activity.
- Cell ImagingVisualize and analyze live or fixed cells using advanced fluorescence.
- Molecular ImagingCutting-edge fluorescent solutions for deep molecular analysis.
- Fluorescence ImagingHigh-resolution imaging solutions for detailed fluorescence studies.
- BioconjugationCustom bioconjugation services for protein, peptide, and dye linking.
- Drug DeliveryTailored fluorescent solutions for efficient drug delivery research.
- Molecular DiagnosticsFluorescent probes and markers for precise molecular diagnostics.
- Flow CytometryFluorescent dyes and reagents for enhanced flow cytometry analysis.
Online Inquiry

