Flow Cytometry - Surface and Intracellular Cell Staining Protocol
Flow cytometry (FCM) is a single-cell–based quantitative analysis and sorting technology performed using a flow cytometer. Flow cytometry is a high-technology platform developed through the advanced integration of monoclonal antibody and immunocytochemistry techniques, laser technology, and computer science. It enables effective discrimination of heterogeneous cell populations at the single-cell level. Detection targets include, but are not limited to, suspended cells, adherent cells, single-cell suspensions dissociated from solid tissues, and other biological particles.
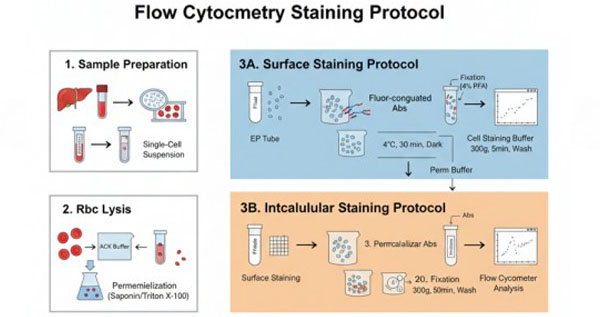 Fig. 1. Flow cytometry cell staining protocol (BOC Sciences Authorized).
Fig. 1. Flow cytometry cell staining protocol (BOC Sciences Authorized).
Materials & Reagents Preparation
Before starting the experiment, please ensure that the following reagents and equipment are prepared. BOC Sciences provides a wide range of high-purity fluorescent dyes and biochemical reagents to support your flow cytometry experiments.
Buffers and Reagents
- Cell staining buffer: PBS containing 1% BSA and 0.1% NaN₃ is recommended (sodium azide inhibits receptor shedding and endocytosis, but should be omitted when sorting live cells).
- Fixative: 4% paraformaldehyde (PFA) or a commercial fixation/permeabilization kit.
- Permeabilization buffer: Buffer containing saponin or Triton X-100.
- Blocking reagent: Anti-mouse/anti-human CD16/32 antibody (Fc Block) or normal homologous serum.
- Viability dyes: Such as PI, 7-AAD, or amine-reactive fluorescent dyes.
Consumables
- 1.5 mL microcentrifuge tubes or 5 mL flow cytometry tubes, centrifuge, micropipettes, and 35–70 μm cell strainers.
Cell Surface Staining Steps
1. Sample Preparation
- a) Collect whole blood or tissues (spleen, lymph nodes, thymus, and bone marrow) and prepare a single-cell suspension using cell staining buffer (or PBS containing 1% BSA). For in vitro–stimulated cells, directly resuspend the stimulated cells in cell staining buffer (or PBS containing 1% BSA), then proceed to step 2.
- b) Fill the tube with cell staining buffer (or PBS containing 1% BSA), centrifuge the cell suspension at 300 g for 5 minutes, and discard the supernatant.
2. Red Blood Cell Lysis
- a) If red blood cell lysis is required (e.g., spleen samples), dilute 10× red blood cell lysis buffer (ACK buffer) to 1× with deionized water and equilibrate to room temperature. Resuspend cells in 3 mL of 1× ACK buffer and incubate at room temperature for 2–3 minutes. If red blood cell lysis is not required, proceed directly to step 3.
- b) Add 10 mL of cell staining buffer (or PBS containing 1% BSA) to stop lysis, centrifuge at 300 g for 5 minutes, and discard the supernatant.
- c) Repeat the wash once by filling to 15 mL with cell staining buffer (or PBS containing 1% BSA), centrifuge at 300 g for 5 minutes, and discard the supernatant.
- d) Count the cells and adjust the concentration to 1 × 10⁷/mL using cell staining buffer (or PBS containing 1% BSA). Aliquot 100 μL of cell suspension into 2 mL microcentrifuge tubes for subsequent use.
3. Fc Receptor Blocking
Blocking Fc receptors reduces nonspecific staining during the staining process:
- a) For mouse samples, purified anti-CD16/CD32 monoclonal antibodies bind FcγRIII/II to block nonspecific staining, reducing background fluorescence of negative cells to the level of unstained cells. Add 0.5–1 μg of purified anti-mouse CD16/32 monoclonal antibody and incubate at room temperature for 10 minutes.
- b) For rat samples, excess purified Ig of the same species and isotype as the fluorescent antibody, serum from the same species, or a commercial Fc receptor blocking reagent may be used.
- c) For human samples, purified anti-human CD16 monoclonal antibody can be used as an Fc receptor blocking reagent. Add 1 μg of purified anti-human CD16 monoclonal antibody and incubate at room temperature for 10 minutes.
4. Cell Staining
- a) Add fluorophore-conjugated antibodies according to the recommended amounts in the datasheet, mix gently, and incubate at 4°C in the dark for 30 minutes.
- b) Add an appropriate volume of cell staining buffer (or PBS containing 1% BSA) to resuspend the cells, centrifuge at 300 g for 5 minutes, and discard the supernatant.
- c) Resuspend the cells in 0.2 mL of cell staining buffer (or PBS containing 1% BSA) and analyze using a flow cytometer.
Notes
- Before use, briefly centrifuge antibody vials to collect contents at the bottom of the tube.
- Fluorophore-conjugated antibodies should be stored at 4°C protected from light and should not be frozen.
- During staining, fixation or delayed analysis may reduce the fluorescence signal of certain antibodies. For optimal results, samples should be analyzed immediately after staining.
Intracellular Cytokine Staining Steps
1. Cell Preparation
- a) Collect cells. After stimulation and blocking treatments (refer to the literature for specific protocols), prepare a single-cell suspension using cell staining buffer (or PBS containing 1% BSA) and adjust the cell concentration to approximately 1 × 10⁷/mL.
- b) Aliquot 100 μL of cell suspension per tube (approximately 1 × 10⁶ cells) into labeled microcentrifuge tubes.
2. Fixation
- a) If surface staining is required, first perform surface staining using appropriate antibodies according to the "Cell Surface Staining Steps."
- b) Resuspend the cells in 1× fixation/permeabilization buffer and incubate at room temperature in the dark for 30 minutes.
- c) Centrifuge at 300 g for 5 minutes and discard the supernatant.
3. Permeabilization
- a) Resuspend the fixed cells in 1× permeabilization buffer, centrifuge at 300 g for 5 minutes, and discard the supernatant.
- b) Repeat the wash once, centrifuge at 300 g for 5 minutes, and discard the supernatant.
- c) Add 1 mL of 1× permeabilization buffer and incubate at 4°C in the dark for 30 minutes; centrifuge at 300 g for 5 minutes and discard the supernatant.
4. Intracellular Staining
- a) Resuspend the cells in 100 μL of 1× permeabilization buffer, add the appropriate antibodies, mix gently, and incubate at 4°C in the dark for at least 30 minutes.
- b) Add 2 mL of 1× permeabilization buffer to resuspend the cells, centrifuge at 300 g for 5 minutes, and discard the supernatant.
- c) Add 2 mL of cell staining buffer (or PBS containing 1% BSA) to resuspend the cells, centrifuge at 300 g for 5 minutes, and discard the supernatant.
- d) Resuspend the cells in 0.2 mL of cell staining buffer (or PBS containing 1% BSA) and analyze using a flow cytometer.
Notes
- When analyzing both surface and intracellular proteins in the same cells, it is recommended to perform surface staining first, followed by fixation and permeabilization.
- Isotype controls are recommended for intracellular staining.
Experimental Controls
Proper control setup is a widely accepted prerequisite for obtaining reliable flow cytometry data and is essential for accurately defining positive populations:
- Blank Control: Cell samples without any fluorescent antibodies. Used to adjust flow cytometer voltages (PMT) and establish baseline background noise.
- Single-Stained Controls: Samples stained with only one fluorophore-conjugated antibody (or compensation beads). Used to calculate the fluorescence compensation matrix and eliminate spectral overlap.
- Isotype Controls: Fluorophore-conjugated antibodies of the same species and isotype as the test antibody but without specific target binding. Primarily used to assess nonspecific binding, especially in intracellular staining.
- FMO Controls (Fluorescence Minus One): In multicolor experiments, samples stained with all antibodies except one fluorophore. Used to define the positive gating boundary for a given channel and eliminate spillover effects from other channels.
Fluorescent Dyes Recommended for Your Research Project
| Catalog | Name | CAS | Inquiry |
|---|---|---|---|
| R10-0005 | 6-Fluorescein phosphoramidite | 204697-37-0 | Bulk Inquiry |
| F04-0033 | 5-Aminofluorescein | 3326-34-9 | Bulk Inquiry |
| F04-0012 | FITC isomer I | 3326-32-7 | Bulk Inquiry |
| A16-0201 | DAPI dihydrochloride | 28718-90-3 | Bulk Inquiry |
| F04-0034 | 5-Carboxyfluorescein diacetate | 79955-27-4 | Bulk Inquiry |
| F04-0055 | Dexamethasone Fluorescein | 216854-76-1 | Bulk Inquiry |
| A16-0033 | 6-Carboxyfluorescein | 3301-79-9 | Bulk Inquiry |
| A16-0004 | Phalloidin-FITC | 915026-99-2 | Bulk Inquiry |
| F02-0030 | Cy3-NHS ester | 146368-16-3 | Bulk Inquiry |
| A16-0074 | Biotin DHPE | 136235-58-0 | Bulk Inquiry |
| A16-0092 | Biotin-XX-SSE | 194041-66-2 | Bulk Inquiry |
| R15-0017 | TCO-PEG4-biotin | 2183440-30-2 | Bulk Inquiry |
| F07-0010 | Biotin-PEG4-Dde-TAMRA-PEG3-Azide | 2353409-56-8 | Bulk Inquiry |
| F07-0016 | Dde TAMRA Biotin Alkyne | 2353409-55-7 | Bulk Inquiry |
| F03-0024 | diSulfo-Cy3 azide | 2055138-89-9 | Bulk Inquiry |
| A19-0101 | Biotin-Aniline | 769933-15-5 | Bulk Inquiry |
Corporate Profile
BOC Sciences serves global research and industry clients, focusing on the development, production, and supply of fluorescent probes, fluorescent dyes, and related customized services. Leveraging a mature chemical synthesis and functional materials platform, the company has established a comprehensive product portfolio that includes small-molecule fluorescent dyes, fluorescent labeling reagents, in vivo imaging probes, and cellular and molecular imaging probes, widely applied in life sciences research, bioimaging, diagnostic development, and materials science.
As a dedicated subsite for fluorescent products, we focus on high-performance fluorescent probes and innovative labeling technologies, offering a diverse selection that includes conventional fluorescent dyes (such as FITC, TRITC, Cy series, Alexa Fluor series, etc.), near-infrared fluorescent probes, environment-responsive probes, and targeted fluorescent molecules. We also support structural modification, spectral tuning, conjugation labeling, and custom synthesis services.
The company has a research and development team composed of experienced chemists and biologists and is equipped with advanced synthesis, purification, and analytical characterization instruments. A strict quality control system ensures that every fluorescent product meets high standards of purity, stability, and optical performance. BOC Sciences' fluorescent probes and related services have been used by universities, research institutions, and biopharmaceutical companies worldwide for cell imaging, molecular tracking, mechanistic studies, and methodological development, with validation and citations in numerous scientific publications. We are committed to being your trusted long-term partner in fluorescent labeling and imaging research.
High-Performance Fluorescent Tools for Your Research
- BODIPYBright, stable dyes used in bioimaging and molecular labeling.
- CoumarinBlue-emitting dyes for chemical sensing and fluorescence studies.
- AF DyesBright, versatile dyes for bioimaging applications.
- Fluorescein FAMGreen fluorescent dye for nucleic acid labeling.
- CyanineVersatile dyes used in biosensing and nucleic acid detection.
- TAMRA DyesRed-emitting dyes for antibody and protein labeling.
- ATTO DyesHigh-performance dyes for labeling and imaging experiments.
- Fluorescent ProteinUsed for live-cell imaging and real-time biosensing.
Custom Fluorescent Solutions
- DNA StainingPrecise fluorescent dyes for clear DNA visualization and analysis.
- Lipid StainingFluorescent solutions for effective lipid structure imaging.
- Cell StainingAdvanced staining for detailed cell morphology and analysis.
- Protein StainingHigh-quality staining for accurate protein detection and imaging.
- Bacteria ImagingFluorescent solutions to visualize bacterial structures and activity.
- Cell ImagingVisualize and analyze live or fixed cells using advanced fluorescence.
- Molecular ImagingCutting-edge fluorescent solutions for deep molecular analysis.
- Fluorescence ImagingHigh-resolution imaging solutions for detailed fluorescence studies.
- BioconjugationCustom bioconjugation services for protein, peptide, and dye linking.
- Drug DeliveryTailored fluorescent solutions for efficient drug delivery research.
- Molecular DiagnosticsFluorescent probes and markers for precise molecular diagnostics.
- Flow CytometryFluorescent dyes and reagents for enhanced flow cytometry analysis.
Online Inquiry

