How Fluorescent-Labeled Antibodies Enhance Live Cell Imaging & Molecular Tracking?
Fluorescent-labeled antibodies (or fluorescent antibodies) play a critical role in cell biology and molecular research by allowing sensitive and specific visualization of target proteins, receptors, and molecular complexes. Paired with fluorophores, these highly specific antibodies have become important reagents in research and have numerous advantages, including real-time tracking of target molecules, minimal interference with normal processes, and multicolor detection of multiple targets within a cell. Complex intracellular dynamic processes can be visualized, and more accurate data is provided for signaling pathway analysis, protein localization, and functional studies. Fluorescent-labeled antibodies are constantly becoming more useful with advancements in microscopy techniques and labeling strategies.
Understanding Fluorescent-Labeled Antibodies
Fluorescently labeled antibodies are conjugates between specific fluorescent compounds (fluorophores) and antibodies that are linked covalently or enzymatically. These conjugates combine the high specificity of antibodies with optical signals that can be read by fluorescence microscopy. This enables the exact localization of the target molecule in the complex environment of the cell.
Direct vs. Indirect Fluorescent Antibody Labeling
Based on the labeling strategy, fluorescent antibodies can be classified as directly labeled antibodies and indirectly labeled antibodies. Directly labeled antibodies attach fluorophores directly to the primary antibody, reducing background signals and being suitable for single-color experiments and rapid detection. Indirectly labeled antibodies use a fluorescent secondary antibody to recognize the unlabeled primary antibody, amplifying signal intensity and enabling detection of low-abundance target proteins or multicolor imaging. Additionally, fluorescent-labeled antibodies can be conjugated with nanoparticles or quantum dots to enhance brightness and photostability, supporting long-term dynamic imaging.
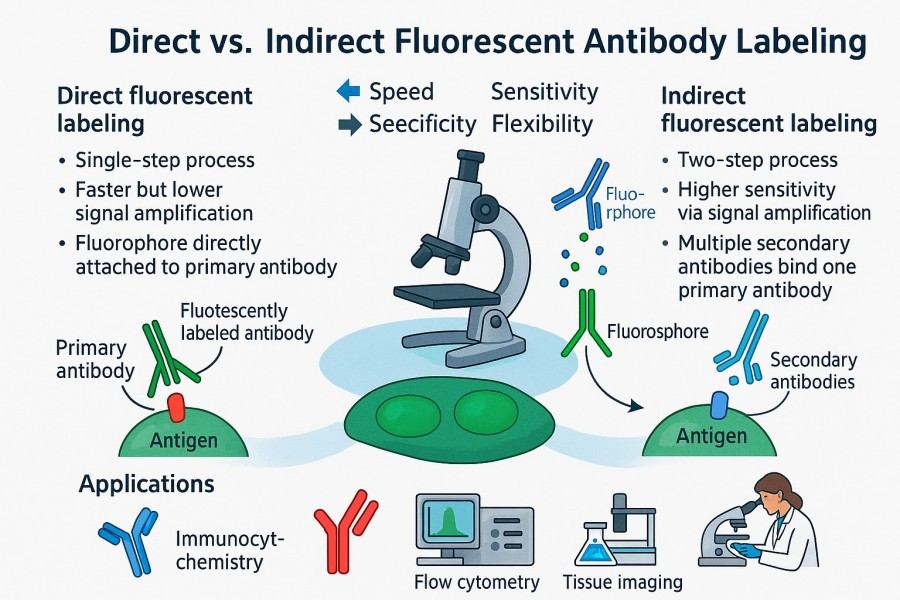 Fig. 1. Directly labeled antibodies and indirectly labeled antibodies (BOC Sciences Authorized).
Fig. 1. Directly labeled antibodies and indirectly labeled antibodies (BOC Sciences Authorized).
Modern fluorescent-labeled antibodies are widely used in flow cytometry, immunofluorescence, live cell imaging, and molecular tracking experiments. Their precise recognition and visualization capabilities make them essential tools for studying protein localization, signaling dynamics, and cellular functions.
How Fluorophores Work in Antibody Labeling?
Fluorophores are the core of antibody visualization. They function by absorbing light at a specific wavelength and emitting light at another wavelength, producing a visible signal. There are numerous types of fluorophores, including common ones such as FITC, TRITC, and the Alexa Fluor series, each with distinct excitation/emission spectra, brightness, and photostability. The choice of fluorophore directly affects experimental quality. For example, FITC emits green light and is suitable for short-term single-color experiments; TRITC or rhodamine series emit red light and can be combined with FITC for multicolor detection; Alexa Fluor series are bright and photostable, ideal for long-term tracking and complex multichannel imaging. Proper fluorophore selection can prevent signal overlap, reduce background noise, and improve detection sensitivity.
Moreover, the conjugation chemistry used to attach fluorophores to antibodies also affects experimental outcomes. Common methods include NHS ester-amine conjugation, thiol-reactive conjugation, and biotin-streptavidin systems, each varying in efficiency, stability, and preservation of antibody activity. A scientifically informed selection of fluorophores and conjugation strategies is key to achieving high-quality live cell imaging.
Key Considerations When Choosing Antibodies for Imaging
Selecting the appropriate antibody is the primary factor in ensuring successful fluorescent imaging experiments. By evaluating these factors comprehensively, researchers can choose fluorescent-labeled antibodies that best suit their experimental needs, enabling high-quality live cell imaging and molecular tracking while providing a reliable basis for subsequent data. Key considerations include:
- Antibody specificity and affinity: High-specificity antibodies ensure accurate recognition of target proteins and avoid background signals from non-specific binding. High-affinity antibodies maintain sensitivity when detecting low-abundance proteins.
- Target protein localization and cell type: Proteins located in the cell membrane, cytoplasm, or nucleus require different permeabilization strategies and fluorescent labeling schemes.
- Experimental type and application needs: Single-color or multicolor imaging, fixed or live cell experiments, real-time tracking or endpoint assays—all influence antibody and fluorophore selection.
- Stability and photobleaching: For long-term imaging or high-intensity experiments, selecting photostable fluorophores and optimizing imaging conditions reduces signal decay.
- Conjugation efficiency and antibody activity preservation: Conjugation may affect antibody recognition. Controlling the fluorophore-to-antibody molar ratio, conjugation conditions, and purification ensures antibody functionality and signal strength.
Advantages of Fluorescent Antibodies in Live Cell Imaging
Observing dynamic processes within live cells is crucial in modern cell biology research. While traditional dyes and chemical labels provide some visualization, they often suffer from high cytotoxicity, low specificity, or rapid signal decay. Fluorescent-labeled antibodies combine highly specific antibodies with stable fluorophores, enabling sensitive, real-time observation of intracellular proteins, receptors, and molecular complexes. Compared to traditional methods, they offer significant advantages in sensitivity, specificity, minimal interference, and multicolor detection, providing a reliable tool for live cell imaging and dynamic molecular tracking.
Real-Time Visualization of Cellular Processes
One of the major advantages of fluorescent-labeled antibodies is the ability to observe dynamic processes in live cells in real time. By coupling antibodies with fluorophores, researchers can track receptor endocytosis and recycling, localization changes of signaling proteins, cytoskeleton remodeling, and organelle movements. For example, during signaling pathway activation, fluorescent antibodies can monitor nuclear-cytoplasmic translocation of key transcription factors, revealing immediate cellular responses to stimuli. This real-time imaging provides not only structural snapshots but also insights into dynamic regulatory mechanisms, making it a core tool for studying complex biological processes.
Additionally, using fluorescent-labeled antibodies in live cells can be combined with high-resolution imaging techniques, such as confocal microscopy, total internal reflection fluorescence (TIRF) microscopy, and super-resolution microscopy, to achieve precise visualization of cellular microstructures and molecular events. This combination allows researchers to observe subtle structural changes undetectable by traditional dyes, providing intuitive data for both basic research and drug development.
High Sensitivity and Specificity in Detection
Fluorescent-labeled antibodies enable highly sensitive detection of low-abundance proteins and rare molecular events, which is difficult with traditional dyes or non-specific probes. Their core advantage lies in the high affinity of antibodies and their highly specific recognition of target antigens. Even when a target protein is expressed only during certain cell cycle phases or microenvironment conditions, fluorescent antibodies provide reliable signals for precise quantification and localization.
By using multicolor fluorescent antibody combinations, researchers can simultaneously detect multiple proteins or molecular complexes, enabling analysis of complex signaling pathways. For instance, in immunological studies, T cell receptor (TCR), co-stimulatory molecules, and signaling proteins can be tracked simultaneously, revealing the temporal and spatial characteristics of immune cell activation. This high-sensitivity, multichannel detection capability is crucial for understanding complex intracellular signaling networks.
Minimal Interference with Cell Function
A key challenge in live cell imaging is maintaining normal cellular physiology during observation. Fluorescent-labeled antibodies, through optimized conjugation ratios and fluorophore selection, can provide strong signals while minimizing interference with cell function. For example, using low molar ratio conjugates or selecting low-toxicity, photostable fluorophores avoids cell stress or functional perturbation caused by antibody binding or illumination.
This minimal interference allows researchers to perform long-term, continuous live cell tracking experiments, yielding data that closely reflect physiological conditions. Properly designed fluorescent antibody experiments can also be combined with other functional assays, such as metabolic activity, migration, or cell cycle analysis, providing multidimensional insights into cell behavior.
Multicolor Imaging and Signal Integration
Fluorescent-labeled antibodies excel not only in single-target detection but also in multicolor imaging and integrated signal analysis. By selecting fluorophores with different excitation and emission wavelengths, multiple molecular events can be tracked simultaneously in the same cell sample, enabling synchronized, multichannel dynamic analysis. This capability is critical for studying signaling networks, protein complex assembly, and cell–cell interactions.
Combined with modern image analysis software, researchers can extract quantitative information from multicolor fluorescence images, including protein co-localization, time-lapse tracking, and single-molecule trajectories. This multidimensional integration makes fluorescent antibodies not only visualization tools but also a vital data source for understanding cell function and molecular mechanisms in live cell imaging.
Improving Molecular Tracking Accuracy with Fluorescent Antibodies
In molecular and cellular studies, merely visualizing static structures is far from sufficient. Scientists are more concerned with the dynamic changes of proteins, molecular complexes, and signaling pathways within live cells. Fluorescent-labeled antibodies, with their high specificity, high sensitivity, and real-time imaging capabilities, have become central tools for molecular tracking experiments. By designing experiments appropriately, fluorescent antibodies can significantly improve tracking accuracy, reduce background interference, and enable simultaneous detection of multiple targets, providing reliable data for analyzing complex intracellular signaling networks.
Tracking Protein Dynamics in Live Cells
Fluorescent antibodies can label key intracellular proteins, enabling real-time tracking of their spatial and temporal distribution. For example, in signaling studies, researchers can use fluorescent antibodies to follow transcription factors moving from the cytoplasm to the nucleus, allowing quantitative analysis of signaling pathway activation rates and durations.
 Fig. 2. Real-time tracking of protein dynamics in live cells using fluorescent-labeled antibodies (BOC Sciences Authorized).
Fig. 2. Real-time tracking of protein dynamics in live cells using fluorescent-labeled antibodies (BOC Sciences Authorized).
In addition, fluorescent-labeled antibodies can be used to observe dynamic interactions of organelle proteins. By labeling mitochondria-, endoplasmic reticulum-, or Golgi-related proteins, researchers can monitor organelle morphological changes and dynamics under stress or drug treatment. This dynamic tracking not only helps reveal molecular mechanisms but also provides critical data for drug target validation and mechanism-of-action studies.
Reducing Background Noise and Signal Spillover
A common challenge in molecular tracking experiments is high background noise or fluorescence signal spillover, especially in multicolor experiments. Fluorescent-labeled antibodies effectively reduce these interferences by:
- Selecting highly specific antibodies: Minimizing non-specific binding to ensure signals originate from target molecules.
- Matching fluorophores with detection channels: Choosing fluorophores with well-separated excitation/emission spectra to avoid channel overlap.
- Optimizing staining and imaging conditions: Controlling antibody concentration, exposure time, and light intensity to minimize background signals and photobleaching.
Combined with modern microscopy techniques and signal analysis software, researchers can further correct channel compensation, achieving precise fluorescence signal separation and quantitative analysis, thereby greatly enhancing tracking accuracy and reliability.
Multiplexing for Simultaneous Detection of Multiple Targets
In complex cellular systems, single-molecule tracking often fails to fully resolve biological processes. Fluorescent-labeled antibodies, through multicolor labeling and multichannel imaging, allow simultaneous tracking of multiple proteins or molecular complexes within the same cell sample. For example, in immune signaling studies, researchers can observe receptors, signaling proteins, and transcription factors simultaneously, revealing spatial and temporal relationships between different molecules.
Multiplex labeling not only improves experimental efficiency but also enables co-localization analysis, molecular interaction studies, and time-lapse dynamic analysis. Combined with quantitative image processing software, researchers can obtain multidimensional data such as protein distribution, movement trajectories, and interaction frequencies, providing comprehensive insights into cellular functions, regulatory mechanisms, and drug effects.
Experimental Optimization Strategies
To achieve high-precision molecular tracking, researchers should consider the following in experimental design:
- Antibody selection and conjugation strategy: Choose appropriate fluorophores and conjugation methods based on target protein expression, cell type, and experiment duration.
- Experimental condition control: Temperature, buffer composition, and imaging intervals affect protein dynamics and should mimic physiological conditions as closely as possible.
- Data correction and analysis: Use software to compensate for channel overlap, remove background noise, and track single-molecule movements for accurate quantitative analysis.
By applying these optimization strategies, the precision and reliability of fluorescent-labeled antibodies in molecular tracking experiments can be significantly enhanced, providing a solid data foundation for cell biology, signaling studies, and drug development.
Common Fluorescent Labels and Their Applications
The successful application of fluorescent-labeled antibodies depends not only on the antibody itself but also on selecting the appropriate fluorophore. Different fluorophores vary in excitation/emission spectra, brightness, photostability, and cellular compatibility. Understanding these properties and choosing fluorophores in line with experimental goals and imaging platforms is key to high-quality live cell imaging and multiplex molecular tracking.
FITC, TRITC, and Alexa Fluor Series
- FITC (fluorescein isothiocyanate) is one of the earliest green fluorophores used for antibody labeling, offering good brightness and suitability for single-color detection. FITC is suitable for short-term imaging experiments, but its photostability is relatively limited and prone to photobleaching under prolonged or intense illumination.
- TRITC (tetramethylrhodamine isothiocyanate) and other rhodamine-series red fluorophores are commonly paired with FITC in multicolor imaging, enabling dual-target detection in green and red channels. Red fluorophores generally offer higher photostability and tissue penetration, facilitating observation of deep structures.
- The Alexa Fluor series is highly bright and photostable, suitable for long-term real-time tracking and high-resolution imaging experiments. Covering excitation/emission wavelengths from UV to far-red, Alexa Fluor dyes support multichannel simultaneous observation and can be combined with other fluorophores for complex multicolor experiments. Researchers can select Alexa Fluor dyes across different wavelengths to achieve optimal signal-to-noise ratio and imaging quality.
Choosing the Right Fluorophore for Your Experiment
When selecting fluorophores, researchers should consider:
- Excitation/emission spectrum matching: Ensure fluorophore wavelengths are compatible with microscope light sources and filters, avoiding spectral conflicts.
- Experiment type: Single-color or multicolor imaging, fixed or live cell experiments, short- or long-term tracking all influence fluorophore choice.
- Brightness and photostability: High brightness and photostable fluorophores are needed for low-abundance targets or long-term dynamic observations.
- Cytotoxicity and compatibility: Select fluorophores with low cytotoxicity and minimal impact on cellular function to maintain live cell health.
By integrating experimental goals, imaging equipment, and cell type, researchers can maximize fluorescence detectability while preserving cell physiology and ensuring reliable results.
Stability and Photobleaching Considerations
Photobleaching, the gradual decay of fluorescence under illumination, is a common issue in imaging experiments, affecting data accuracy. To improve experimental stability, researchers can:
- Choose highly photostable fluorophores, such as the Alexa Fluor series or modified far-red dyes.
- Reduce excitation light intensity and exposure time to minimize fluorophore damage while maintaining signal strength.
- Use anti-photobleaching reagents in culture or imaging buffers to extend fluorescence lifespan.
- Optimize imaging frequency, especially for long-term dynamic tracking, to reduce cumulative light exposure effects.
Proper fluorophore selection and photobleaching management ensure stable and reliable fluorescence signals in long-term and multicolor imaging experiments. This not only improves the data quality of live cell imaging but also provides a solid foundation for multiplex molecular tracking and dynamic analysis.
Common Fluorescent Reagents for Antibody Labeling
| Catal | Name | CAS | Color | Ex (nm) | Em (nm) | Applications | Inquiry |
|---|---|---|---|---|---|---|---|
| F04-0012 | FITC isomer I (Fluorescein 5-isothiocyanate) | 3326-32-7 | Yellow-green | ~ 498 nm | ~ 517 nm | Protein/antibody labeling, flow cytometry, immunofluorescence | Bulk Inquiry |
| F02-0026 | Cy5-NHS ester | 146368-14-1 | Far-red | ~ 649 nm | ~ 670 nm | Labeling of proteins, antibodies, oligonucleotides; flow cytometry, FRET, microscopy | Bulk Inquiry |
| F02-0030 | Cy3-NHS ester | 146368-16-3 | Orange | ~ 555 nm | ~ 570 nm | Amino-group labeling (proteins, peptides, antibodies), immunofluorescence, flow cytometry | Bulk Inquiry |
| A16-0093 | Rhodamine 6G | 989-38-8 | Yellow-orange | ~ 525–530 nm | ~ 548 nm | Laser dye, P-gp efflux assays, mitochondrial probe, flow cytometry, fluorescence microscopy | Bulk Inquiry |
| A01-0005 | Rhodamine B | 81-88-9 | Red / Pink | ~ 546 nm | ~ 568 nm | Fluorescent staining (e.g. auramine-rhodamine stain), microscopy, flow cytometry, tracer dye | Bulk Inquiry |
| F04-0036 | Fluorescein isothiocyanate-dextran | 60842-46-8 | Green | ~ 490–495 nm | ~ 515–520 nm | Tracking/diffusion studies, cell permeability, endocytosis assays, live cell imaging (dextran conjugate) | Bulk Inquiry |
| A16-0019 | Tetramethylrhodamine isothiocyanate (TRITC, mixed isomers) | 95197-95-8 | Orange-red | ~ 550–555 nm (typical for TRITC) | ~ 570–580 nm (typical for TRITC) | Immunofluorescence, labeling of proteins/antibodies, microscopy, FACS | Bulk Inquiry |
| R01-0438 | Cy5-NHS ester tetrafluoroborate | 1263093-76-0 | Far-red | ~ 650–650+ nm | ~ 670 nm | Antibody conjugation, flow cytometry, multicolor imaging | Bulk Inquiry |
| R01-0469 | AF647 NHS ester (Alexa Fluor 647) | 1620475-28-6 | Far-red | ~ 650 nm | ~ 665–670 nm | Antibody labeling, flow cytometry, super-resolution imaging, immunofluorescence | Bulk Inquiry |
| A03-0016 | Oregon Green 488 carboxylic acid | 195136-52-8 | Green | ~ 488 nm (Oregon Green 488) | ~ 515–525 nm | Conjugation to biomolecules, live cell imaging, pH sensors, fluorescent probes | Bulk Inquiry |
Looking for Antibody Labeling Dyes?
We provide flexible conjugation options with various fluorophores, including water-soluble and photostable dyes, to meet your experimental requirements.
Tips for Optimizing Live Cell Imaging Experiments
The success of live cell imaging experiments depends not only on high-quality fluorescent antibodies but also on optimization across experimental design, imaging parameters, and sample handling. Even the best fluorescent-labeled antibodies can produce weak signals, high background, or impaired cell function if experimental conditions are not properly controlled. By optimizing antibody concentration, imaging settings, and sample preparation workflows, researchers can acquire high signal-to-noise ratio and high-resolution images while maintaining cell health.
Optimizing Antibody Concentration
Antibody concentration is a key factor influencing fluorescence signal intensity and background noise. Excessive antibody can cause non-specific binding and increased background signals, compromising precise localization, while insufficient antibody may fail to label the target protein effectively, resulting in weak or undetectable signals.
Methods for optimizing antibody concentration include:
- Gradient concentration testing: Conduct preliminary experiments with a range of concentrations to identify the optimal signal-to-noise ratio.
- Consider target protein abundance: High-abundance proteins may require lower antibody concentrations, whereas low-abundance or weak-signal targets may need slightly higher concentrations.
- Coordinate with conjugation ratio: The molar ratio of fluorophore to antibody directly affects brightness and background and should be optimized alongside antibody concentration.
By adjusting antibody concentration appropriately, researchers can achieve high-specificity labeling, low background signals, and uniform fluorescence distribution, providing reliable data for downstream imaging analysis.
Correct Imaging Settings and Instrument Calibration
Imaging settings are another critical factor for successful live cell experiments. Whether using confocal microscopy, widefield microscopy, or super-resolution systems, proper parameter settings can significantly improve image quality.
Key considerations include:
- Excitation intensity and exposure time: Excessive light or prolonged exposure can cause photobleaching and cell damage; adjust based on fluorophore properties and cell type.
- Filter and channel selection: Use filters matching the excitation/emission wavelengths of fluorophores to avoid signal spillover.
- Microscope calibration: Regularly calibrate optical paths, correct aberrations, and adjust focal planes to ensure resolution and uniform signal.
- Multichannel compensation: In multicolor imaging, use software or hardware compensation to reduce spectral overlap and improve signal accuracy.
Proper imaging settings and instrument calibration not only enhance single-cell signal detectability but also provide a reliable foundation for multichannel, time-lapse dynamic tracking experiments.
Sample Preparation Best Practices
Sample quality directly affects the reliability and reproducibility of live cell imaging. High-quality sample preparation balances cell viability, antibody penetration, and signal uniformity.
Key strategies include:
- Maintain cell health: Use appropriate culture medium, temperature, and CO₂ conditions to avoid excessive stress.
- Buffer selection: Choose low-background, imaging-compatible buffers that do not affect fluorophore stability or antibody binding.
- Staining and incubation conditions: Control antibody incubation time and temperature to ensure uniform labeling without overbinding.
- Minimize cell disturbance: Handle cells gently to prevent detachment or damage, ensuring even signal distribution.
- Rapid imaging: Capture images soon after labeling to reduce signal decay and changes in cell state.
By strictly controlling sample preparation workflows, researchers can obtain fluorescence signals that reflect true physiological conditions, providing reliable data for live cell imaging while minimizing experimental errors.
BOC Sciences' Expertise in Fluorescent-Labeled Antibody Services
With years of biochemical research and development experience, BOC Sciences offers one-stop professional services from fluorophore synthesis and antibody conjugation to experimental optimization. Whether for multicolor imaging, real-time dynamic tracking, or low-abundance protein detection, we provide customized solutions and strict quality control to deliver stable, efficient, and reliable experimental tools, supporting high-level scientific research and innovative applications.
Support for Synthesis and Modification of Various Fluorophores
- Synthesis and chemical modification services for FITC, TRITC, Alexa Fluor series, and other customizable fluorophores to meet diverse experimental needs.
- Optimization of fluorophore photostability, brightness, and solubility based on client requirements to ensure compatibility with different imaging platforms.
- Functionalization of fluorophores, such as introducing reactive groups, PEGylation, or special derivatives, to enhance conjugation efficiency and antibody compatibility.
- Spectral optimization for multicolor and long-term live cell imaging, ensuring clear signals with minimal interference.
Custom Fluorescent Antibody Conjugation Services
- Efficient covalent conjugation of primary or secondary antibodies, balancing antibody specificity and fluorescence signal intensity.
- Optimization of fluorophore-to-antibody molar ratio and conjugation conditions based on target protein expression and experimental design.
- Support for monoclonal, polyclonal, and nanobody conjugation, accommodating diverse research directions and applications.
- Custom fluorescent antibody combinations for multicolor, multi-target labeling.
One-Stop Live Cell Imaging Solutions
- Comprehensive experimental design services covering antibody selection, conjugation, labeling, and imaging.
- Recommendations for optimal fluorophores, antibody concentrations, and imaging parameters to achieve high signal-to-noise ratio.
- Support for live cell dynamic tracking, multichannel co-localization, and long-term imaging experiments to ensure reliable data.
- Technical guidance and optimization advice, including photobleaching management, sample handling, and microscope parameter adjustment.
Quality Control and Optimized Labeling Conditions
- Strict quality control for each batch of fluorescent-labeled antibodies, including labeling efficiency, fluorescence brightness, and specificity validation.
- Stability testing and photobleaching assessment to ensure signal consistency in long-term and multicolor experiments.
- Optimized conjugation conditions to reduce non-specific binding and background noise, enhancing experimental reliability.
- Detailed product information and usage guidance to help researchers quickly perform high-quality live cell imaging experiments.
Do You Need A Consultation?
BOC Sciences integrates cutting-edge fluorescence technologies to accelerate your research, driving next-generation solutions for drug discovery and diagnostics.
References
- Vira S, et al. Fluorescent-labeled antibodies: Balancing functionality and degree of labeling. Anal Biochem. 2010; 402(2):146–150. DOI: 10.1016/j.ab.2010.03.036. PMID: 20362543.
- Dean KM, et al. Advances in fluorescence labeling strategies for dynamic cellular imaging. Nat Chem Biol. 2014; 10(7):512–523. DOI: 10.1038/nchembio.1556. PMID: 24937069.
- Wang W, et al. Real-time imaging of cell-surface proteins with antibody-based fluorogenic probes. Chem Sci. 2021; 12(40):13477–13482. DOI: 10.1039/d1sc03065e. PMID: 34777767.
Transform Your Studies with Cutting-Edge Fluorescent Products
| Catalog | Name | CAS | Inquiry |
|---|---|---|---|
| A16-0159 | HIDC iodide | 36536-22-8 | Bulk Inquiry |
| A19-0040 | Hoechst 33342 | 23491-52-3 | Bulk Inquiry |
| A16-0201 | DAPI dihydrochloride | 28718-90-3 | Bulk Inquiry |
| A16-0005 | MitoMark Green I | 201860-17-5 | Bulk Inquiry |
| A16-0036 | Calcein Blue | 54375-47-2 | Bulk Inquiry |
| A16-0156 | Speed DiI | 278173-35-6 | Bulk Inquiry |
| A16-0084 | Speed DiO | 164472-75-7 | Bulk Inquiry |
| A19-0060 | Hoechst 34580 | 23555-00-2 | Bulk Inquiry |
| A14-0005 | Fluo-4 AM | 273221-67-3 | Bulk Inquiry |
| A14-0008 | Fura-2 AM | 108964-32-5 | Bulk Inquiry |
High-Performance Fluorescent Tools for Your Research
- TAMRA Dyes Red-emitting dyes for antibody and protein labeling.
- ATTO Dyes High-performance dyes for labeling and imaging experiments.
- Coumarin Blue-emitting dyes for chemical sensing and fluorescence studies.
- Fluorescent Protein Used for live-cell imaging and real-time biosensing.
More About Antibody labeling
Online Inquiry

