Comparing Rhodamine Derivatives: Rhodamine 6G, Rhodamine B, Rhodamine 123 and More
Rhodamine dyes, as a class of classic fluorescent dyes, are widely used in fields such as bioimaging, flow cytometry, sensing probes, and materials science due to their high brightness, excellent photostability, and versatile chemical modification capabilities. With the increasing demands of modern research, scientists are paying more attention to the differences among various rhodamine derivatives in terms of spectral properties, labeling efficiency, and biocompatibility. This article systematically compares commonly used rhodamine derivatives, including Rhodamine 6G, Rhodamine B, Rhodamine 123, and other important derivatives, providing in-depth analysis on structural features, application scenarios, advantages and limitations, and experimental considerations, offering authoritative guidance and practical reference for researchers selecting and applying rhodamine dyes.
Introduction to Rhodamine Derivatives
In the fields of fluorescent dyes, imaging agents, sensing probes, and optical materials within biochemistry and materials science, rhodamine compounds have become essential tools due to their outstanding optical properties, chemical modifiability, and highly controllable labeling capabilities. As a typical class of xanthene dyes, rhodamine derivatives exhibit strong visible-light absorption and high quantum yield fluorescence emission, demonstrating excellent performance in experiments ranging from fundamental research to advanced biomedical imaging.
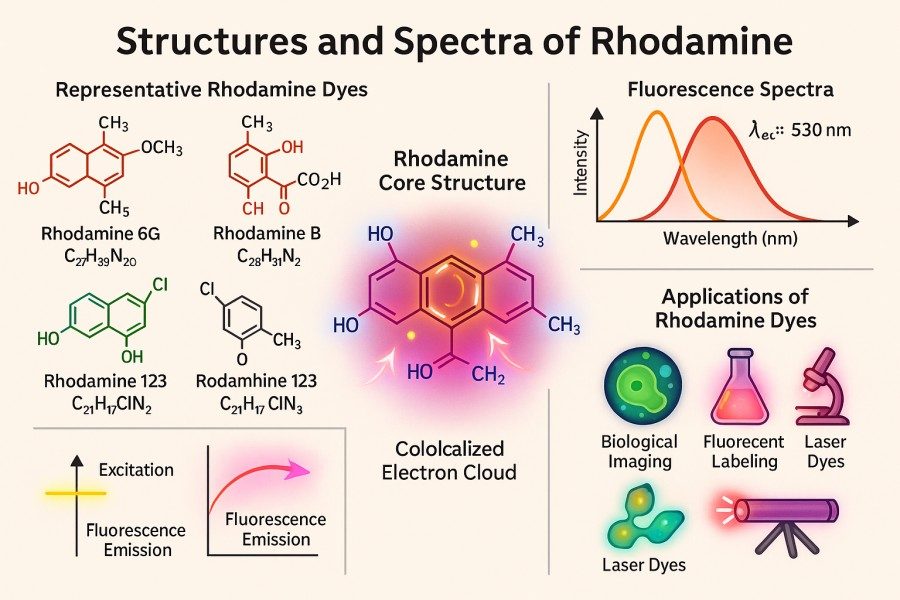 Fig. 1. Structures and spectra of Rhodamine (BOC Sciences Authorized).
Fig. 1. Structures and spectra of Rhodamine (BOC Sciences Authorized).
What are Rhodamine Derivatives?
Rhodamine derivatives are fluorescent dyes based on the xanthene core structure, with functional groups such as amino, hydroxyl, carboxyl, and others introduced on the benzene, pyran, or pyridine rings. These molecules typically feature a highly conjugated π-electron system, allowing efficient absorption of visible-light photons and subsequent fluorescence emission. Differences among derivatives are mainly reflected in the type of substituents, molecular charge properties, hydrophilicity/hydrophobicity, and reactive groups available for bioconjugation.
To enable labeling of biomacromolecules, nanomaterials, or synthetic polymers, rhodamine derivatives often incorporate reactive groups into the parent structure, such as isothiocyanate (–N=C=S), activated esters, amino, carboxyl, or click chemistry groups. These chemical modifications not only ensure efficient covalent binding to target molecules but also allow flexible use across various experimental systems, including immunofluorescence staining, nucleic acid probe labeling, and membrane protein tracking.
Importance of Rhodamine Dyes in Fluorescence Research
Rhodamine dyes play a central role in fluorescence research due to their high brightness, tunable spectra, and strong chemical stability. Their excitation and emission wavelengths cover the visible spectrum from green to red, enabling flexible multiplexing in imaging experiments while maintaining high signal-to-noise ratios. In bioimaging, rhodamine derivatives are widely used for organelle staining, protein tracking, live-cell monitoring, and mitochondrial membrane potential assessment. In flow cytometry and fluorescence-activated cell sorting (FACS), these dyes are preferred for antibody labeling and multicolor experiments due to their high brightness and stability. Additionally, rhodamine dyes are valuable in environmental sensing, chemical detection, and materials science applications such as laser dyes and optical sensors.
Despite their advantages, practical use of rhodamine dyes requires attention to photobleaching, cytotoxicity, phototoxicity, and background signal interference. Researchers should consider the experimental system, excitation light intensity, dye concentration, and conjugation strategy to ensure reliable and reproducible results.
Overview of Common Rhodamine Derivatives
The diversity of rhodamine derivatives allows them to meet different experimental needs in fluorescence labeling, imaging, and sensing research. Variations in excitation/emission spectra, brightness, photostability, chemical modifiability, and biocompatibility make choosing the right dye critical for optimizing experimental outcomes and signal-to-noise ratio. The following provides a systematic analysis of several commonly used rhodamine derivatives.
TRITC – Properties, Applications, and Labeling Uses
TRITC (Tetramethylrhodamine Isothiocyanate) is a rhodamine derivative commonly used for protein and antibody labeling. Its molecular structure contains an isothiocyanate (–N=C=S) group that covalently couples with amino groups of lysine residues in proteins. TRITC has an excitation peak around 550–555 nm and an emission peak around 570–590 nm, offering good brightness and high photostability. TRITC is widely applied in immunofluorescence staining, antibody labeling, cell membrane and organelle tracking experiments. Its labeling reactions occur under mild conditions and maintain stable fluorescence across a broad pH range, demonstrating good compatibility in various biological systems. Moreover, TRITC can be combined with other fluorescent dyes for multicolor imaging, providing flexible solutions for complex cell and molecular tracking studies.
| Catalog | Name | CAS | Inquiry |
|---|---|---|---|
| A16-0002 | Phalloidin-TRITC | 915013-10-4 | Bulk Inquiry |
| A16-0019 | Tetramethylrhodamine isothiocyanate (mixed isomers) | 95197-95-8 | Bulk Inquiry |
TAMRA – Key Features and Fluorescent Applications
TAMRA (Tetramethylrhodamine Amide) is a rhodamine derivative featuring a carboxyl-terminal group, allowing for further modification and conjugation. It has an excitation peak around 550 nm and an emission peak around 575–585 nm. TAMRA is highly water-soluble, bright, and can be covalently coupled to antibodies, peptides, oligonucleotides, and other molecules through esterification or activated ester chemistry. In fluorescent probe development, TAMRA is commonly used in FRET (Fluorescence Resonance Energy Transfer) systems, molecular sensors, and multicolor live-cell imaging. It should be noted that TAMRA may aggregate at high concentrations or in lipid-rich environments, leading to fluorescence quenching. Optimizing dye concentration and buffer conditions is therefore essential to maintain stable signals during experiments.
| Catalog | Name | CAS | Inquiry |
|---|---|---|---|
| F07-0012 | TAMRA-PEG3-Azide | 1228100-59-1 | Bulk Inquiry |
| F07-0021 | TAMRA-PEG4-acid | 1909223-02-4 | Bulk Inquiry |
| F07-0017 | TAMRA-PEG4-Alkyne | 1225057-68-0 | Bulk Inquiry |
| F07-0029 | TAMRA-PEG3-biotin | 2279944-59-9 | Bulk Inquiry |
| F07-0048 | TAMRA DBCO, 5-isomer | 1911598-65-6 | Bulk Inquiry |
| F07-0001 | TAMRA-PEG4-t-butyl ester | 2353409-64-8 | Bulk Inquiry |
| F07-0004 | TAMRA-PEG2-Maleimide | 2304558-24-3 | Bulk Inquiry |
| F07-0006 | TAMRA-PEG4-DBCO | 1895849-41-8 | Bulk Inquiry |
| F07-0009 | TAMRA-Azide-PEG-Biotin | 1797415-74-7 | Bulk Inquiry |
Rhodamine B – Characteristics, Typical Uses, and Conjugation Options
Rhodamine B is a classic red fluorescent dye with an excitation peak around 568 nm and an emission peak around 583 nm. It is bright, cost-effective, and chemically stable, making it suitable for water flow tracing, staining experiments, and basic fluorescence research. Rhodamine B can be conjugated to proteins or peptides via activated ester or isothiocyanate groups, but it tends to aggregate in aqueous solutions, which may cause fluorescence quenching. Its derivatives are widely used in the preparation of fluorescent probes, antibody labeling, and small molecule tagging, making Rhodamine B a popular economical choice in research applications.
| Catalog | Name | CAS | Inquiry |
|---|---|---|---|
| A14-0036 | Rhodamine B hydrazide | 74317-53-6 | Bulk Inquiry |
| A16-0171 | Octadecyl Rhodamine B Chloride | 65603-19-2 | Bulk Inquiry |
| A01-0005 | Rhodamine B | 81-88-9 | Bulk Inquiry |
| A14-0059 | N-hydroxy Rhodamine B amide | 1115867-62-3 | Bulk Inquiry |
| A14-0060 | Rhodamine B thiospirolactone | 111883-10-4 | Bulk Inquiry |
| A16-0149 | Rhodamine B hexyl ester perchlorate | 877933-92-1 | Bulk Inquiry |
Rhodamine 6G – Properties, Brightness, and Research Applications
Rhodamine 6G is a highly bright rhodamine derivative with a quantum yield up to 0.95. It has an excitation peak around 525 nm and an emission peak around 548–556 nm, ranking among the top in fluorescence brightness and photostability within the rhodamine series. Rhodamine 6G is widely employed as a laser dye, in water flow tracing, fluorescence microscopy, and high signal-to-noise ratio experiments. Its high brightness and low photobleaching characteristics make it ideal for long-term excitation experiments or single-molecule imaging, making it an excellent choice for high-sensitivity fluorescence assays.
| Catalog | Name | CAS | Inquiry |
|---|---|---|---|
| A17-0093 | Rhodamine 6G tetrafluoroborate | 54854-14-7 | Bulk Inquiry |
| A16-0093 | Rhodamine 6G | 989-38-8 | Bulk Inquiry |
| A17-0016 | Rhodamine 6G Perchlorate | 13161-28-9 | Bulk Inquiry |
| A16-0142 | Dihydrorhodamine 6G | 217176-83-5 | Bulk Inquiry |
Rhodamine 123 – Unique Features and Experimental Applications
Rhodamine 123 is a cationic, partially lipophilic dye that selectively accumulates in mitochondria and is used to monitor membrane potential. It has an excitation peak around 507 nm and an emission peak around 529 nm. Rhodamine 123 is commonly applied in live-cell imaging, mitochondrial function assessment, and drug activity studies. Although its brightness is slightly lower than Rhodamine 6G, its specificity for organelle localization gives it unique advantages in cellular research. Care should be taken to minimize photobleaching and cytotoxicity, which may affect experimental outcomes.
| Catalog | Name | CAS | Inquiry |
|---|---|---|---|
| A16-0170 | Rhodamine-123 | 62669-70-9 | Bulk Inquiry |
| A03-0012 | Dihydrorhodamine 123 | 109244-58-8 | Bulk Inquiry |
Rhodamine 110 – Properties, Cell Imaging, and Bioconjugation
Rhodamine 110 has an excitation peak around 488 nm and an emission peak around 520–530 nm, making it suitable for multicolor fluorescence labeling experiments. It is highly water-soluble, and its terminal reactive groups facilitate covalent conjugation with proteins, peptides, or small molecule probes. Rhodamine 110 is widely used for antibody labeling, multicolor live-cell imaging, and molecular probe development. When combined with other dyes, it effectively reduces spectral overlap and background interference, but proper filter selection is essential to optimize the signal-to-noise ratio.
| Catalog | Name | CAS | Inquiry |
|---|---|---|---|
| A18-0008 | Rhodamine 110 chloride | 13558-31-1 | Bulk Inquiry |
| F05-0007 | Carboxyrhodamine 110-PEG4-alkyne | 2055103-66-5 | Bulk Inquiry |
| F05-0006 | Carboxyrhodamine 110-PEG3-Azide | 1536327-95-3 | Bulk Inquiry |
Key Challenges When Using Rhodamine Dyes
Although rhodamine dyes offer high brightness, broad spectral coverage, and good chemical stability in fluorescence research, several challenges remain in practical applications. These issues can directly impact the reliability, reproducibility, and accuracy of experimental results. Researchers must carefully consider these factors when designing experiments and selecting dyes to ensure accurate and actionable fluorescence signals.
- Photostability and Photobleaching Issues: Photobleaching is the gradual loss of a dye's fluorescence emission under intense or prolonged excitation. Even photostable dyes like Rhodamine 6G can experience bleaching under high-power excitation or single-molecule imaging conditions.
- Signal to Noise Ratio Optimization: Background fluorescence, autofluorescence, dye aggregation, quenching effects, and buffer interference can all reduce signal intensity. Strategies to optimize the signal-to-noise ratio include careful filter selection, background reduction, prevention of dye self-aggregation, and optimization of signal acquisition parameters.
- Cytotoxicity and Phototoxicity Concerns: In live-cell imaging, dye toxicity and phototoxicity must be considered. Some rhodamine dyes may pose toxicity risks in aqueous or cellular environments, and high-intensity excitation can lead to oxidative stress and cell death.
- Compatibility with Biological Systems: Dye compatibility varies across different biological systems. Factors such as solvent, buffer composition, staining efficiency, membrane permeability, conjugation efficiency, and compatibility with other labeled dyes must be taken into account.
Strategies to Overcome Challenges in Rhodamine Dye Applications
To address issues like photobleaching, reduced signal-to-noise ratio, cytotoxicity, and compatibility, researchers can adopt a series of strategies to optimize experimental design and improve fluorescence imaging quality and reproducibility. By selecting the right dye, optimizing labeling conditions, using advanced imaging technologies, and incorporating protective agents, these challenges can be effectively mitigated.
Selecting the Right Rhodamine Derivative for Your Experiment
Choosing an appropriate rhodamine derivative is the first step in solving experimental challenges. Researchers should consider:
- Excitation/Emission Spectrum: Avoid overlap with sample autofluorescence or other dyes to enhance resolution in multicolor imaging.
- Photostability and Brightness: For long-term imaging or single-molecule detection, select dyes with high photostability and quantum yield (e.g., Rhodamine 6G).
- Conjugation Functional Groups: Choose reactive groups such as isothiocyanate, activated ester, or click chemistry moieties for efficient covalent labeling.
- Biocompatibility: For live-cell experiments, select low-toxicity dyes with moderate membrane permeability to avoid interfering with cell function.
Scientific evaluation of dye properties and experimental requirements can minimize photobleaching and signal interference from the outset.
Optimizing Dye Concentration and Labeling Conditions
Dye concentration and labeling conditions directly affect fluorescence intensity, uniformity, and background signal. Optimization strategies include:
- Start with low concentrations and gradually adjust to achieve optimal signal while avoiding dye aggregation or self-quenching.
- Control conjugation time and temperature to ensure sufficient labeling without excessive reaction, minimizing non-specific binding.
- Use appropriate buffer systems to prevent high salt or organic solvents from reducing dye solubility or causing quenching.
- In multicolor experiments, employ cross-validation or sequential staining to reduce spectral interference between dyes.
Proper dye usage strategies can significantly improve signal-to-noise ratio while minimizing potential cytotoxic effects.
Advanced Imaging Techniques to Improve Signal Detection
Advanced imaging methods can further enhance fluorescence signal quality, mitigating photobleaching and background interference. Common techniques include:
- Confocal Microscopy: Removes out-of-focus background via optical sectioning, improving spatial resolution.
- Fluorescence Lifetime Imaging (FLIM): Distinguishes signals based on fluorescence lifetime rather than intensity, reducing background interference.
- Time-Gated Fluorescence Detection: Filters out short-lived background fluorescence by delayed signal acquisition.
- Super-Resolution Imaging: Techniques such as STED, SIM, or PALM/STORM enable high-precision observation at the single-molecule level.
These technologies enhance data reliability and resolution in live-cell imaging, multicolor labeling, and low-signal experiments.
Use of Protective Agents and Antifade Reagents
Antifade reagents and protective agents are effective for extending dye fluorescence lifetime and improving experimental reproducibility. Common strategies include:
- Adding oxygen radical scavengers (e.g., Trolox, β-mercaptoethanol) to reduce photoxidation damage.
- Using antifade mounting media to limit continuous excitation damage.
- Controlling excitation intensity and exposure time, combined with intermittent illumination, to reduce photofatigue.
- Adjusting pH and ionic strength in dye solutions or staining systems to maintain structural stability.
Proper use of protective agents preserves rhodamine dye brightness and signal stability under prolonged imaging or high-power excitation.
Looking for Rhodamine Dyes?
Whether you require specialized Rhodamine derivatives, water-soluble variants, or photostable forms, our experts offer tailored synthesis solutions to meet your research and experimental needs.
Insights into Rhodamine Dye Synthesis and Quality Considerations
The high performance and experimental reliability of rhodamine dyes depend not only on molecular design but also on synthesis processes, purity control, and batch consistency. Researchers should understand preparation methods, quality metrics, and potential customization options to ensure accurate and reproducible results.
Common Synthetic Routes for Rhodamine Derivatives
Rhodamine derivatives are generally synthesized based on the xanthene core, with functionalization of the aromatic rings to tune optical and chemical properties. Key strategies include:
- Acid-Catalyzed Condensation: Condensation of diaryl aldehydes or phenol derivatives with aromatic amines under acidic conditions forms the xanthene core, followed by substitution reactions to yield different derivatives.
- Substituent Modification: Introduction of hydroxyl, amino, carboxyl, or isothiocyanate groups regulates water solubility, membrane permeability, and bioconjugation capability.
- Silicon or Near-Infrared Modification: Replacing oxygen with silicon (Si-Rhodamine) or adding conjugated groups shifts excitation and emission to red/NIR regions for deep tissue imaging or multicolor experiments.
- Reactive Group Incorporation: Terminal groups such as activated esters, isothiocyanates, or click chemistry moieties enable covalent conjugation to proteins, nucleic acids, or small molecules.
Different synthetic routes determine photophysical performance, chemical stability, and biocompatibility, guiding researchers in selecting the most suitable derivative.
Purity and Batch-to-Batch Consistency Impact on Research Outcomes
Dye purity and batch consistency directly affect experimental results. Impurities or heterogeneous chemical composition can cause:
- Fluorescence intensity fluctuations, impacting quantitative analysis and multicolor imaging comparability.
- Increased background signals, reducing signal-to-noise ratio and complicating data interpretation.
- Reduced conjugation efficiency, affecting antibody or molecular probe labeling.
High purity (>95%) and strict quality control are critical for research-grade rhodamine dyes. Common quality tests include HPLC, LC-MS, and NMR to ensure structural and purity accuracy. Researchers should review supplier quality reports and batch consistency statements.
Custom Synthesis Options for Specialized Research Needs
As experimental requirements become more complex, standard rhodamine dyes may not fully meet specific needs. Custom synthesis offers flexible solutions, including:
- Adjusting excitation/emission wavelengths for specific multicolor or near-infrared imaging.
- Introducing specialized reactive groups (click chemistry, photo-controllable groups) for advanced conjugation or sensor design.
- Optimizing water solubility, membrane permeability, or biocompatibility for live-cell or in vivo imaging experiments.
Custom synthesis enhances dye performance and ensures compatibility with specific molecular systems, supporting cutting-edge research.
Applications of Rhodamine Derivatives Across Research Fields
Rhodamine derivatives play a key role in biomedical research, chemical analysis, environmental monitoring, and materials science due to their high brightness, tunable spectra, and chemical modifiability. Understanding their applications allows researchers to design experiments that maximize fluorescence signal reliability and reproducibility.
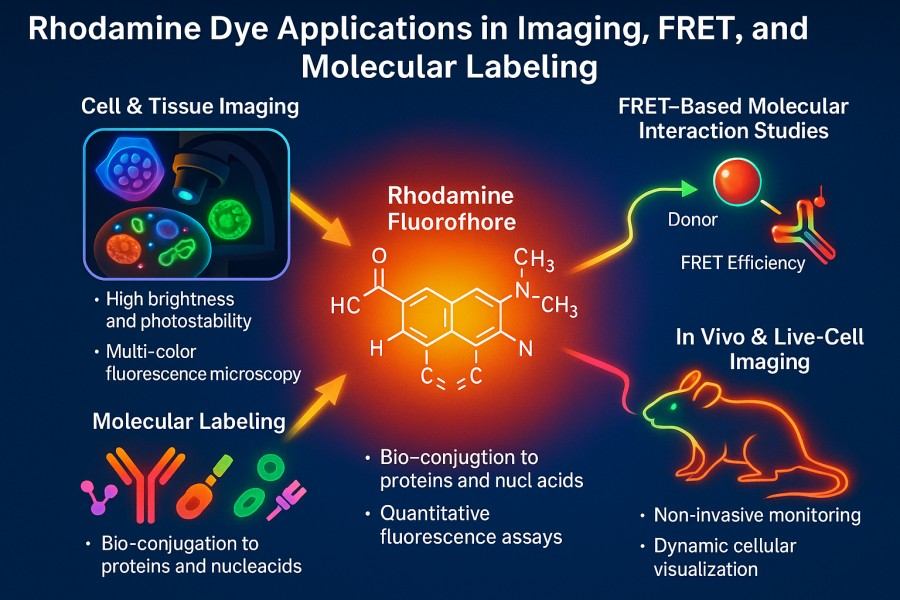 Fig. 2. Rhodamine dye applications in imaging, FRET, and molecular labeling (BOC Sciences Authorized).
Fig. 2. Rhodamine dye applications in imaging, FRET, and molecular labeling (BOC Sciences Authorized).
Cellular and Molecular Imaging
Rhodamine dyes are widely used for organelle labeling, protein tracking, and live-cell monitoring. For example, Rhodamine 123 selectively accumulates in mitochondria to assess membrane potential and function, while TRITC and TAMRA are commonly used for antibody labeling and multicolor confocal imaging. High brightness and tunable spectra enable long-term imaging, and careful dye combinations allow simultaneous observation of multiple targets, enhancing experimental data dimensionality.
Flow Cytometry and Fluorescence-Activated Cell Sorting (FACS)
Rhodamine derivatives are widely used in antibody labeling and multiplex staining due to their high brightness and photostability. Conjugated to surface or intracellular proteins, they enable precise classification, functional analysis, and dynamic tracking of cell populations. For example, Rhodamine B and Rhodamine 6G-labeled antibodies combined with FACS allow high-throughput cell analysis. Spectral differences among rhodamine derivatives reduce signal interference in multicolor experiments, improving sorting accuracy and reliability.
Environmental and Chemical Sensing
Rhodamine derivatives are valuable in environmental monitoring and chemical sensing. Their fluorescence signals can detect metal ions, pH, solvent polarity, and small molecule concentrations. Rhodamine 6G and Rhodamine B are often used for water quality and heavy metal detection, providing high-sensitivity readouts. Chemically modified derivatives can function as responsive sensors, exhibiting fluorescence switching or intensity changes upon binding specific ions or molecules, enabling dynamic and repeatable environmental and chemical monitoring.
Clinical Diagnostics and Biomarker Detection
In clinical diagnostics and biomarker detection, rhodamine dyes are used in immunoassays, fluorescence in situ hybridization (FISH), microarray analysis, and in vitro diagnostic (IVD) reagents. Their high brightness and chemical stability enhance sensitivity and accuracy. For example, TAMRA and Rhodamine 110 can label oligonucleotide probes for rapid detection of specific nucleic acid sequences. Multicolor imaging allows simultaneous monitoring of multiple biomarkers, supporting high-throughput, quantitative clinical testing for disease diagnosis and biological research.
Through widespread applications, rhodamine derivatives not only enhance fluorescence imaging, analytical detection, and sensing technologies but also provide multifunctional, reliable tools for research experiments.
Why Choose BOC Sciences for Your Rhodamine Dye Needs?
For both research and industrial applications, selecting high-quality, stable rhodamine dyes is crucial for experimental results and efficiency. BOC Sciences offers a comprehensive portfolio of rhodamine derivatives, flexible custom synthesis services, and professional technical support to help researchers and industry clients achieve optimal performance in fluorescence labeling and imaging experiments.
Comprehensive Rhodamine Dye Portfolio
- Offers a wide range of commonly used and specialized rhodamine derivatives, including TRITC, TAMRA, Rhodamine B, Rhodamine 6G, Rhodamine 123, and Rhodamine 110.
- Each batch undergoes strict purity and batch consistency testing to ensure high brightness, stability, and reliable optical performance.
- Provides pre-labeled or unlabeled dyes according to customer needs, facilitating flexible experiment design.
Custom Synthesis and Modification Services
- Offers custom synthesis, including tuning excitation/emission wavelengths, introducing specific conjugation groups, or adjusting hydrophilicity/hydrophobicity.
- Provides molecular modification and functionalization, including carboxyl, amino, isothiocyanate, and click chemistry groups.
- Flexibly supports advanced research and industrial applications, meeting requirements for patent development, functional optimization, and special experimental conditions.
Rhodamine Labeling and Conjugation Support
- Offers efficient covalent conjugation for antibodies, proteins, peptides, and nucleic acids.
- Optimizes conjugation conditions to ensure high labeling efficiency, low background, and reproducible results.
- Supports multicolor imaging, FRET systems, and high-throughput assays, facilitating complex experimental workflows.
Technical Support for Optimized Dye Application
- Provides comprehensive technical guidance on dye selection, labeling conditions, imaging parameters, and antifade strategies.
- Offers customized experimental optimization for different systems and research needs, improving efficiency.
- Leverages biochemical and materials science expertise to solve technical challenges in experiments.
Do You Need A Consultation?
BOC Sciences integrates cutting-edge fluorescence technologies to accelerate your research, driving next-generation solutions for drug discovery and diagnostics.
Transform Your Studies with Cutting-Edge Fluorescent Products
| Catalog | Name | CAS | Inquiry |
|---|---|---|---|
| A14-0036 | Rhodamine B hydrazide | 74317-53-6 | Bulk Inquiry |
| F05-0031 | 6-Carboxy-X-rhodamine | 194785-18-7 | Bulk Inquiry |
| A17-0069 | Rhodamine 590 Chloride | 3068-39-1 | Bulk Inquiry |
| A17-0106 | Rhodamine 19 Perchlorate | 62669-66-3 | Bulk Inquiry |
| A16-0014 | Sulforhodamine 101 | 60311-02-6 | Bulk Inquiry |
| A17-0061 | Rhodamine 610 Perchlorate | 23857-51-4 | Bulk Inquiry |
| A17-0107 | Rhodamine 640 Perchlorate | 72102-91-1 | Bulk Inquiry |
| A17-0047 | Rhodamine 700 perchlorate | 63561-42-2 | Bulk Inquiry |
| A17-0016 | Rhodamine 6G Perchlorate | 13161-28-9 | Bulk Inquiry |
| A14-0060 | Rhodamine B thiospirolactone | 111883-10-4 | Bulk Inquiry |
High-Performance Fluorescent Tools for Your Research
- TAMRA Dyes Red-emitting dyes for antibody and protein labeling.
- ATTO Dyes High-performance dyes for labeling and imaging experiments.
- Coumarin Blue-emitting dyes for chemical sensing and fluorescence studies.
- Fluorescent Protein Used for live-cell imaging and real-time biosensing.
More About Rhodamine Dyes
Online Inquiry

