How to Optimize Rhodamine Conjugation Efficiency for Better Results?
Rhodamine, as a high-brightness and stable fluorescent dye, plays a crucial role in biomolecular labeling and fluorescence imaging. However, achieving efficient Rhodamine conjugation depends not only on the quality of the dye itself but also on the choice of conjugation strategies, experimental conditions, and subsequent storage methods. This article provides an in-depth exploration of the concept, strategies, challenges, and optimization methods of Rhodamine conjugation, helping researchers achieve efficient labeling of antibodies, proteins, peptides, nucleic acids, and lipids to obtain reliable experimental results.
What is Rhodamine-Conjugated?
Rhodamine conjugation is a core technique widely applied in modern molecular biology and biomedical research. It involves covalently or enzymatically attaching Rhodamine fluorescent dyes to target biomolecules. Through this approach, researchers can achieve highly sensitive detection and real-time imaging at the cellular or molecular level, enabling precise observation of the dynamic changes and spatial distribution of biomolecules. Rhodamine conjugation not only enhances the visibility of experimental signals but also provides reliable analytical tools for complex biological systems, making it an important technique in cell biology, immunology, and drug development.
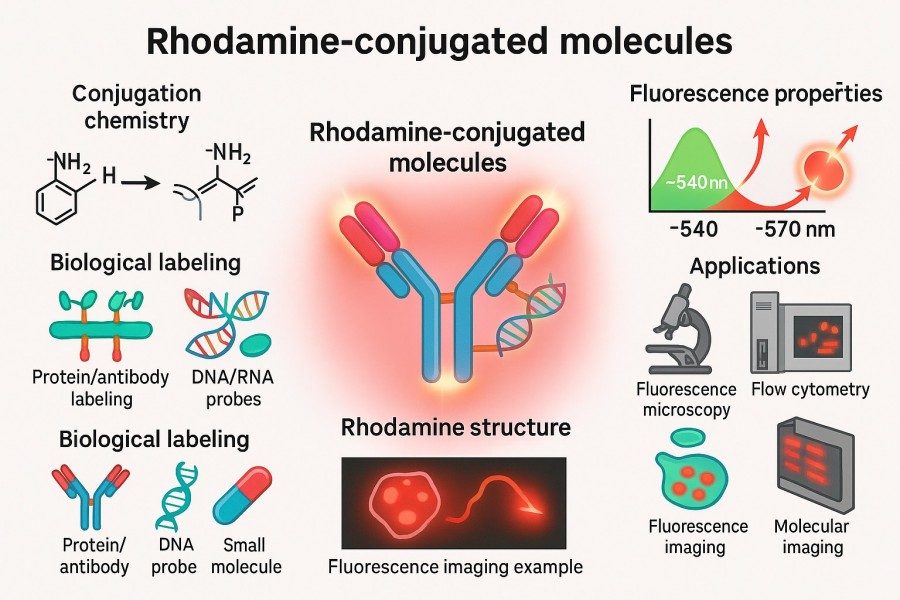 Fig. 1. Rhodamine-conjugated molecules (BOC Sciences Authorized).
Fig. 1. Rhodamine-conjugated molecules (BOC Sciences Authorized).
Definition and Applications of Rhodamine-Conjugated Molecules
Rhodamine-conjugated molecules refer to complexes in which Rhodamine dyes are covalently linked or strongly associated through non-covalent interactions with biomolecules such as proteins, antibodies, peptides, nucleic acids, or lipids. These conjugates tightly couple fluorescent signals with biological functions, allowing precise labeling. Rhodamine-conjugated molecules have wide-ranging research and application value, including but not limited to:
- Fluorescence microscopy imaging: Used to visualize organelle localization, protein distribution, and dynamic transport processes, helping to elucidate cellular functions and signaling mechanisms.
- Flow cytometry (FACS): Enables quantitative analysis of surface markers and intracellular signals of specific cell populations, making high-throughput cell analysis more efficient.
- Fluorescence immunoassays (ELISA, Western Blot): Combined with antigen–antibody specificity, these assays enable highly sensitive quantitative detection, suitable for studying protein expression and interactions.
- Fluorescence in situ hybridization (FISH): Used to localize specific DNA or RNA sequences, providing direct visualization tools for gene expression and chromosomal analysis.
- Fluorescent probes and signal transduction studies: By labeling small molecules or signaling proteins, researchers can track intracellular signaling pathway activation and dynamic changes in real time.
Through Rhodamine labeling, researchers can achieve precise molecular-level localization and dynamic monitoring, significantly enhancing data reliability, reproducibility, and visualization—thereby providing strong support for life science research.
Advantages of Rhodamine Labeling in Fluorescent Imaging and Assays
Rhodamine dyes are widely used in fluorescent labeling due to their outstanding properties:
- High fluorescence quantum yield: Rhodamine dyes produce bright and intense fluorescent signals upon excitation, enabling clear detection of even trace target molecules.
- Excellent photostability: Rhodamine shows strong resistance to photobleaching under standard imaging and analytical conditions, ensuring stable signals over long experimental periods.
- Compatibility with multichannel imaging: The emission wavelength of Rhodamine allows it to be combined with dyes such as FITC and Cy5, supporting multiplex labeling while minimizing signal overlap and interference.
- Stable and modifiable chemical structure: Rhodamine molecules are chemically stable and can be efficiently conjugated through NHS esters, maleimide chemistry, or click chemistry, compatible with proteins, antibodies, peptides, and nucleic acids.
These features ensure that Rhodamine-conjugated molecules deliver reliable and reproducible fluorescent signals in basic research, drug development, and biomolecular labeling applications, providing a strong foundation for high-precision experimental design and data analysis. By scientifically optimizing conjugation strategies, Rhodamine labeling can achieve optimal performance in imaging sensitivity, labeling stability, and experimental reproducibility.
Looking for Rhodamine Dyes?
Whether you need fluorescently labeled proteins, peptides, antibodies, or other biomolecules, our experts can provide Rhodamine products tailored to your research needs.
Key Rhodamine Conjugation Strategies: Applications and Techniques
The choice of Rhodamine conjugation strategy directly affects labeling efficiency, signal intensity, and the preservation of biomolecular function. Different types of biomolecules—such as antibodies, proteins, peptides, nucleic acids, and lipids—have distinct requirements for conjugation methods. A well-chosen strategy can enhance fluorescence signal stability and uniformity while maintaining the biological functionality of the target molecules. This section systematically introduces Rhodamine conjugation techniques and their applications for different biomolecular targets.
Rhodamine-Conjugated Antibody
Rhodamine dyes functionalized with reactive groups—such as carboxyl, isothiocyanate, or PEG moieties—can covalently couple to amino or thiol groups on antibodies, forming stable fluorescent conjugates without disrupting antigen recognition.
Applications:
- Immunohistochemistry (IHC): Enables precise localization of target proteins within tissue sections or cellular samples, generating high-contrast fluorescent images.
- Flow Cytometry: Facilitates identification and quantification of specific cell populations in multiparameter analyses.
- Enzyme-Linked Immunosorbent Assay (ELISA): Provides sensitive detection of antigens or antibodies in microplate-based quantitative assays.
Technical Advantages:
- Preserves antibody binding activity through mild conjugation conditions.
- Delivers strong, stable fluorescence ideal for detecting low-abundance targets.
- Compatible with multiplexing for simultaneous detection of multiple analytes.
Rhodamine-Conjugated Protein
Rhodamine labeling of proteins allows covalent attachment at selected amino acid residues, generating targeted fluorescent probes for mechanistic and structural studies.
Applications:
- Protein dynamics: Monitoring folding, degradation, and conformational changes through fluorescence intensity or lifetime measurements.
- Protein–protein interactions: Using FRET or fluorescence polarization to study complex formation, dissociation, and kinetics.
- Mechanistic investigations: Tracking protein localization and functional changes in live cells or in vitro systems.
Technical Advantages:
- Enables site-specific labeling at amino or thiol groups.
- Maintains native protein activity due to gentle modification.
- Compatible with fluorescence microscopy, flow cytometry, and high-throughput screening platforms.
Rhodamine-Conjugated Peptide
Peptide labeling with Rhodamine creates versatile fluorescent probes for structural and functional studies. A notable example is Rhodamine-conjugated phalloidin, widely used to stain F-actin filaments with high specificity and brightness.
Applications:
- Cytoskeletal visualization: Rhodamine–phalloidin provides sharp, photostable labeling of actin filaments in fixed or live cells.
- Peptide tracking: Enables real-time visualization of peptide uptake, distribution, and localization.
- Structural studies: Facilitates fluorescence-based assays for conformational changes or peptide–protein interactions.
Technical Advantages:
- High specificity and strong signal-to-noise ratio for actin imaging.
- Stable labeling suitable for extended imaging sessions.
- Compatible with multiplex staining strategies in cell biology.
Rhodamine-Conjugated Nucleic Acid (DNA/RNA)
Rhodamine dyes can be chemically linked to nucleic acids at terminal positions, backbone sites, or specific bases, yielding highly sensitive fluorescent probes.
Applications:
- DNA/RNA probe development: Used in FISH, qPCR probes, and real-time nucleic acid detection.
- Molecular interaction studies: Enables analysis of protein–nucleic acid binding, folding, and structural dynamics via FRET and related techniques.
- Gene expression and cell tracking: Tracks transcription, transport, and degradation of labeled nucleic acids in living cells.
Technical Advantages:
- High brightness and photostability for long-term monitoring.
- Specific modification at 3′/5′ ends or bases for targeted labeling.
- Compatible with other dyes for multiplexed nucleic acid imaging.
Rhodamine-Conjugated Lipid
Rhodamine-conjugated lipids are essential tools for membrane studies, enabling visualization of lipid distribution, dynamics, and interactions.
Applications:
- Cell membrane imaging: Fluorescent lipids integrate into membranes for high-resolution visualization of membrane structures and protein distribution.
- Lipid dynamics: Monitoring membrane fluidity, endocytosis/exocytosis, and liposome behavior.
- Drug delivery research: Tracking liposomes or lipid nanoparticles in nanocarrier systems.
Technical Advantages:
- High-intensity, photostable fluorescence for extended imaging.
- Compatible with multicolor labeling for complex membrane studies.
- Flexible conjugation to various lipid classes for stable membrane insertion.
Rhodamine-Conjugated Streptavidin
Streptavidin labeled with Rhodamine enables robust biotin-based detection and purification strategies.
Applications:
- Biotin detection: Visualization of biotinylated proteins, nucleic acids, or other biomolecules with high affinity and specificity.
- Multiplex assays: Combines with biotin–avidin systems in ELISA, Western blotting, and immunoprecipitation.
- Biosensing: Integration into biosensor platforms for signal amplification.
Technical Advantages:
- Retains high biotin-binding capacity post-labeling.
- Provides intense fluorescence for sensitive detection.
- Compatible with diverse assay formats and labeling systems.
Rhodamine-Conjugated Dextran
Dextran conjugated with Rhodamine is a widely used tracer for studying molecular transport, cell permeability, and tissue architecture.
Applications:
- Tracer studies: Mapping diffusion, vascular permeability, and intercellular transport.
- Cell tracking: Monitoring uptake and intracellular distribution in live cells.
- Neuroscience and developmental biology: Visualizing transport in tissues and extracellular spaces.
Technical Advantages:
- High water solubility and biocompatibility.
- Uniform labeling for consistent fluorescence intensity.
- Suitable for in vivo and in vitro applications across multiple model systems.
Key Challenges in Rhodamine Conjugation
Although Rhodamine conjugation has been widely applied in the labeling of antibodies, proteins, peptides, and nucleic acids, numerous challenges remain in practical applications. If not properly addressed, these issues can directly affect labeling efficiency, fluorescence signal intensity, and the functional stability of biomolecules. Scientists must fully understand and avoid these challenges when designing conjugation experiments to ensure reliable and reproducible results.
Maintaining Fluorescence and Signal Stability
Fluorescence stability is one of the core indicators in Rhodamine conjugation experiments. Photobleaching and fluorescence quenching are among the most common problems during labeling, often leading to rapid signal decay or signal heterogeneity. To maintain high signal stability, it is recommended to avoid direct exposure to intense light during handling, use light-protective materials or work under red light, and add antioxidants such as ascorbic acid or Trolox to the buffer to reduce oxidative loss. In addition, optimizing the pH and ionic strength of the buffer helps stabilize fluorescence, ensuring that labeled molecules emit consistent and uniform signals throughout the experiment.
Avoiding Over-Labeling and Steric Hindrance
Over-labeling can compromise molecular function and reduce signal reliability. Excess Rhodamine may cause steric hindrance by occupying surface space on the target molecule, preventing interaction with ligands or antigens. Furthermore, the active sites of proteins or antibodies may be masked by dyes, leading to functional loss. To avoid these problems, it is essential to control the molar ratio of conjugation scientifically, select appropriate labeling sites, and prioritize site-specific labeling strategies to balance functional preservation and signal stability.
Ensuring Biocompatibility and Specificity
Ensuring the biocompatibility and specificity of labeled molecules is equally critical during Rhodamine conjugation. Non-specific binding or improper chemical modifications may increase background signals, reduce experimental sensitivity, and cause signal instability. Harsh conditions such as high temperature or strong acids and bases can also lead to molecular degradation or inactivation. To ensure compatibility, mild reaction conditions should be chosen, buffer systems optimized, and purification techniques applied to remove unbound dyes, resulting in highly specific and stable Rhodamine conjugates.
Rhodamine Conjugation Strategies for High Labeling Efficiency
Achieving high labeling efficiency in Rhodamine conjugation relies not only on the photostability and reactivity of the dye itself but also on selecting the appropriate conjugation chemistry and optimizing reaction conditions based on the characteristics of the target molecule. Through systematic design and precise control, conjugation efficiency can be significantly improved, non-specific labeling reduced, and strong, low-background, and highly reproducible fluorescent conjugates obtained.
Choosing the Right Functional Groups (NHS Esters, Maleimide, etc.)
Different Rhodamine derivatives often carry various reactive groups for covalent conjugation to biomolecules such as antibodies, proteins, and peptides. Selecting the correct reactive group is a key step to achieving efficient and stable labeling:
- NHS esters (N-hydroxysuccinimide esters): NHS esters react rapidly with primary amines on lysine residues of proteins to form stable amide bonds. This reaction is mild and highly efficient, suitable for labeling most proteins and antibodies. However, it is a random labeling method and may affect certain functional regions of the molecule.
- Maleimide: Maleimide groups specifically react with thiol groups on cysteine residues to form stable thioether bonds. This highly specific reaction is ideal for site-specific labeling of cysteine residues, avoiding disruption of the molecule's native conformation and activity.
- Imide/Click Chemistry: For labeling strategies requiring precise control, click chemistry or other highly selective imide-based reactions can be employed. These reactions have strong selectivity and minimal side reactions, making them suitable for biological imaging and quantitative analysis where high spatial resolution and uniform signals are required.
Optimizing Reaction Conditions (pH, Temperature, and Concentration)
Labeling efficiency depends not only on the choice of functional groups but also strongly on the physicochemical conditions of the reaction system. Proper control of pH, temperature, and reactant concentrations can significantly enhance labeling efficiency without damaging the biomolecule:
- pH control: The optimal pH for NHS ester conjugation is typically between 7.0 and 8.0, where amine nucleophilicity is higher, promoting stable amide bond formation. Acidic conditions reduce reactivity, while overly basic conditions can cause dye or protein degradation.
- Temperature control: Reaction temperature should be carefully managed. Performing reactions at room temperature or 4 °C helps avoid protein denaturation or dye hydrolysis at higher temperatures. For slow reactions, extending the reaction time is preferable to increasing the temperature.
- Concentration ratio: The molar ratio between the dye and the target molecule should be optimized through pilot experiments. Excess dye may lead to multipoint labeling, fluorescence quenching, or conformational interference, whereas insufficient dye results in low labeling efficiency and unstable signals.
Site-Specific vs Random Labeling: Which to Choose?
Depending on the application, Rhodamine labeling strategies can be divided into random and site-specific labeling:
- Random labeling: Involves labeling through lysine or other common amino acid residues. This approach is simple and broadly applicable, but the lack of fixed labeling sites can cause reduced protein activity or uneven signal intensity.
- Site-specific labeling: Involves introducing specific reactive sites (e.g., engineered cysteine residues) to achieve precise conjugation. This approach preserves molecular functionality to the greatest extent and produces highly uniform and quantifiable fluorescent signals, making it ideal for high-precision bioanalysis, single-molecule detection, and diagnostic applications.
Purification Methods to Remove Unbound Rhodamine
After conjugation, unbound Rhodamine dyes must be completely removed to prevent high background signals or non-specific binding in subsequent experiments. Common purification methods include:
- Size-exclusion chromatography (SEC): Separates labeled products from free dyes based on molecular size differences. It is simple to perform and suitable for rapid purification of most antibody and protein conjugates.
- Ultrafiltration: Uses molecular weight cut-off (MWCO) membranes to remove low-molecular-weight unbound dyes through repeated centrifugation and dilution steps, suitable for small-scale experiments.
- High-performance liquid chromatography (HPLC): For products requiring high purity and precise labeling control, HPLC provides high resolution and is suitable for quantitative analysis and preparation of highly pure conjugates.
Best Practices for Long-Term Stability of Rhodamine Conjugates
Rhodamine fluorescent dyes are widely used for labeling antibodies, proteins, nucleic acids, and other biomolecules due to their high brightness, excellent photostability, and versatility. However, improper storage or handling can cause fluorescence signals to decay over time or even lead to structural degradation of the molecules, resulting in unstable or irreproducible experimental outcomes. By following proper storage strategies and handling practices, the lifespan of Rhodamine conjugates can be significantly extended, maintaining signal strength and molecular integrity.
Storage Conditions and Buffer Selection
Rhodamine conjugates are sensitive to temperature, light, oxidation, and pH fluctuations during storage. Therefore, establishing appropriate storage conditions is essential:
- Light protection and low-temperature storage: Rhodamine molecules are photosensitive, and prolonged exposure to strong light can cause irreversible damage to fluorescent groups. Conjugates should be stored in light-protected containers (e.g., amber centrifuge tubes or foil-wrapped containers) at 4 °C or −20 °C to minimize fluorescence decay and molecular degradation. For long-term storage, it is recommended to aliquot and freeze the samples to avoid repeated freeze–thaw cycles.
- Buffer selection: Storage buffers should maintain pH stability, biocompatibility, and protect the dye. Commonly used buffers include PBS (pH 7.4) or HEPES. Avoid buffers containing primary amines or thiols (e.g., Tris or buffers containing DTT), as they may react with conjugation groups and lead to bond cleavage.
- Addition of preservatives and antioxidants: To prevent microbial contamination, low concentrations of preservatives (e.g., 0.02% sodium azide) can be added to storage buffers. Including small amounts of antioxidants (e.g., ascorbic acid or TCEP) helps slow oxidation and improve conjugate stability. However, it is crucial to ensure that these additives do not compromise fluorescence performance or biological activity.
Avoiding Photobleaching During Experiments
Photobleaching is a common issue in fluorescence experiments, particularly under high-intensity excitation or during prolonged imaging. Proper experimental design and handling can effectively reduce photobleaching and extend signal detection time:
- Control light intensity and exposure time: Avoid continuous strong illumination of samples. Intermittent exposure or reducing excitation intensity during imaging can help minimize irreversible signal loss caused by overexcitation of fluorescent molecules.
- Select appropriate excitation wavelengths and filters: Using laser/LED sources and filter sets that match Rhodamine's excitation and emission spectra improves signal-to-noise ratios while minimizing damage from off-peak excitation energy.
- Use antifade reagents: For long-term imaging or real-time tracking experiments, antifade buffers or mounting media containing antioxidants can be added to effectively slow Rhodamine photobleaching and maintain stable signals over extended periods.
High-Quality Rhodamine Conjugation Services and Expert Custom Support
Leveraging extensive expertise in organic synthesis and bioconjugation technologies, BOC Sciences offers comprehensive Rhodamine conjugation services, supporting diverse labeling schemes for antibodies, proteins, peptides, and nucleic acids. We tailor optimal conjugation strategies based on experimental needs and molecular characteristics to ensure high labeling efficiency, strong fluorescence signals, and excellent bioactivity. Our experienced technical team can handle complex systems with flexibility, providing high-quality fluorescent labeling solutions for research, diagnostics, imaging, and drug development.
Rhodamine–Antibody Conjugation Services
- Offers both random and site-specific labeling strategies to preserve antibody activity and ensure high-affinity binding.
- Multiple Rhodamine dye types are available to match various experimental platforms and excitation/emission wavelength requirements.
- Rigorous purification and quality control ensure high purity and excellent signal-to-noise ratios.
- Adjustable labeling ratios are supported to meet diverse applications ranging from basic research to industrial-scale production.
- Custom solutions are provided to optimize conjugation conditions based on antibody type and experimental objectives, achieving optimal labeling performance.
Rhodamine–Protein Conjugation Services
- Tailored conjugation strategies are designed according to protein characteristics (molecular weight, structure, functional group distribution) to improve labeling efficiency.
- Employs NHS esters, Maleimide, or Click Chemistry for efficient and stable Rhodamine conjugation.
- Preserves the native structure and functionality of proteins, avoiding inactivation or unstable signals caused by over-labeling.
- Supports both research-scale and industrial-scale production for use in scientific research, drug development, and pharmaceutical manufacturing.
- Fluorescence intensity and purity are strictly tested to ensure stable and reliable performance under various experimental conditions.
Rhodamine–Peptide Conjugation Services
- Adapts labeling strategies to different peptide lengths and structures (linear or cyclic) to ensure functional preservation.
- Supports multiple labeling and specialized functional modifications to meet the needs of high-throughput or complex experimental designs.
- High-purity preparations ensure uniform, strong, and stable fluorescence signals, enhancing imaging and analytical outcomes.
- Applicable to a wide range of applications including cell imaging, signaling pathway studies, and targeted delivery.
- Custom conjugation strategies are provided based on peptide sequences and experimental goals to optimize results.
Rhodamine–Nucleic Acid Conjugation Services
- Offers precise labeling site selection (5′ end, 3′ end, or internal modifications) to preserve nucleic acid functionality.
- High-purity preparations ensure strong probe specificity, high detection sensitivity, and reduced background signals.
- Suitable for various research and diagnostic applications, including FISH, real-time PCR, molecular probes, and nucleic acid detection.
- Supports conjugation for DNA, RNA, and PNA, accommodating diverse experimental needs.
- Provides flexible customization of labeling ratios and batch sizes for both research-grade and industrial-scale production.
Do You Need A Consultation?
BOC Sciences integrates cutting-edge fluorescence technologies to accelerate your research, driving next-generation solutions for drug discovery and diagnostics.
Transform Your Studies with Cutting-Edge Fluorescent Products
| Catalog | Name | CAS | Inquiry |
|---|---|---|---|
| A16-0170 | Rhodamine-123 | 62669-70-9 | Bulk Inquiry |
| A14-0036 | Rhodamine B hydrazide | 74317-53-6 | Bulk Inquiry |
| A17-0093 | Rhodamine 6G tetrafluoroborate | 54854-14-7 | Bulk Inquiry |
| A16-0171 | Octadecyl Rhodamine B Chloride | 65603-19-2 | Bulk Inquiry |
| F05-0026 | ROX alkyne, 6-isomer | 2097422-22-3 | Bulk Inquiry |
| F05-0028 | ROX azide, 6-isomer | 1422178-12-8 | Bulk Inquiry |
| F05-0030 | TR-X-NHS ester, 5-isomer | 178623-11-5 | Bulk Inquiry |
| F05-0001 | ROX azide, 5-isomer | 2628213-67-0 | Bulk Inquiry |
| F05-0010 | R110 azide, 6- isomer | 1622395-29-2 | Bulk Inquiry |
| F05-0006 | Carboxyrhodamine 110-PEG3-Azide | 1536327-95-3 | Bulk Inquiry |
High-Performance Fluorescent Tools for Your Research
- BODIPY Bright, stable dyes used in bioimaging and molecular labeling.
- Coumarin Blue-emitting dyes for chemical sensing and fluorescence studies.
- Cyanine Versatile dyes used in biosensing and nucleic acid detection.
- Fluorescent Protein Used for live-cell imaging and real-time biosensing.
More About Rhodamine Dyes
Online Inquiry

