Rhodamine Synthesis: Overcoming Challenges in Fluorescent Applications
Rhodamine dyes have become commonly used fluorescent reagents in scientific research and industry due to their excellent fluorescence performance, photostability, and broad applicability. In fields such as bioimaging, fluorescent probe design, and optoelectronic materials, the high brightness and tunable spectral properties of Rhodamine provide strong support for experiments. However, the synthesis of Rhodamine involves multiple chemical methods and technical routes, and the optimization of each step directly affects the dye's purity, stability, and optical properties. This article provides a comprehensive overview of Rhodamine synthesis strategies, key techniques, application directions, and best laboratory practices, aiming to offer researchers a systematic reference to achieve efficient and reproducible Rhodamine preparation and application.
Understanding Rhodamine and Its Role in Fluorescence
In modern biochemical and molecular imaging research, fluorescent dyes are widely used as signal probes to achieve highly sensitive detection and real-time monitoring of complex biological systems. Among the numerous fluorescent molecules, Rhodamine holds a highly important position due to its unique optical properties and high chemical tunability. Since the 20th century, Rhodamine dyes have been continuously developed and improved, becoming not only a classic choice for molecular probe design but also demonstrating irreplaceable value in immunoassays, cell tracking, optical sensors, and nanotechnology applications.
What is Rhodamine?
Rhodamine is a class of organic fluorescent dyes based on the xanthene backbone, typically forming various derivatives through amino substitution and carboxyl modification (such as Rhodamine 6G, Rhodamine B, Rhodamine 123, etc.). Upon excitation, they can emit strong fluorescence ranging from orange-red to green, making them widely used in biological labeling and optical probes. The emission range of Rhodamine typically spans 500–600 nm, which aligns well with common laser sources such as argon-ion and helium-neon lasers, making it a widely used high-sensitivity fluorescent marker in laboratories. Compared with other traditional fluorescent dyes (e.g., fluorescein and indole dyes), Rhodamine exhibits higher fluorescence quantum yield and better photostability, allowing it to maintain stable signal output under prolonged excitation, significantly enhancing experimental data reliability. Moreover, the molecular structure of Rhodamine provides ample space for chemical modification, enabling precise tuning of excitation/emission wavelengths, solubility, and targeting properties by introducing different substituents, thereby meeting diverse research and industrial needs.
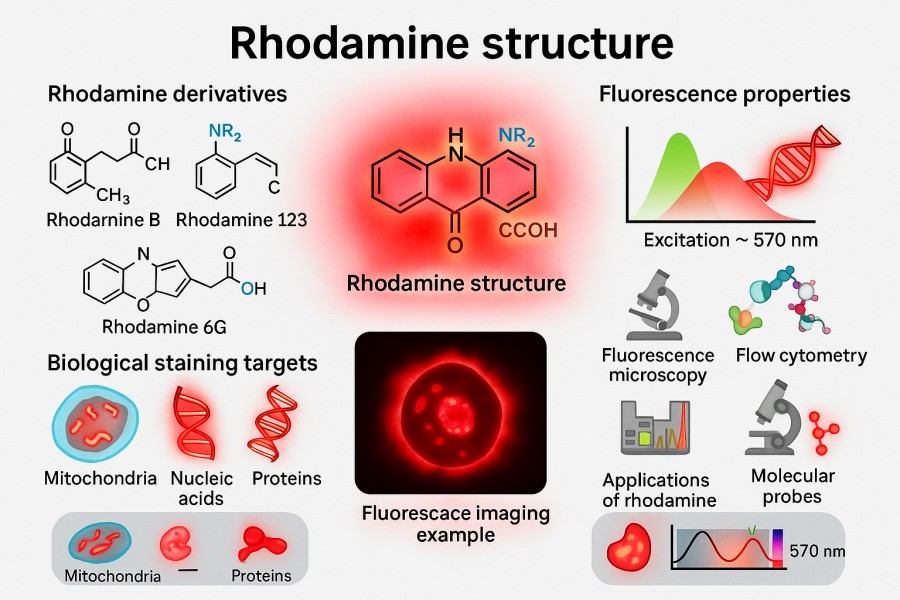 Fig. 1. Rhodamine structure (BOC Sciences Authorized).
Fig. 1. Rhodamine structure (BOC Sciences Authorized).
Unique Photophysical Properties of Rhodamine Dyes
Rhodamine dyes offer the following core advantages in biological applications:
- High quantum yield: Efficiently converts excitation light into fluorescence, enhancing detection sensitivity, especially for low-abundance molecules.
- Broad absorption spectrum and strong emission: Can be excited over a wide wavelength range and emit intense fluorescence, compatible with various optical devices.
- Excellent photostability: Maintains consistent signals during long-term imaging and real-time monitoring, reducing experimental errors caused by photobleaching.
- Good water solubility and cell permeability: Facilitates use in biological systems, enabling penetration through cell membranes and localization in specific organelles.
- Structural modifiability: Functional groups can be introduced to enhance hydrophilicity, provide targeting capability, or bind specific biomolecules.
Looking for Rhodamine Dyes?
Whether you require specialized Rhodamine derivatives, water-soluble variants, or photostable forms, our experts offer tailored synthesis solutions to meet your research and experimental needs.
Rhodamine Synthesis: Key Insights for Researchers and Developers
Research on Rhodamine synthesis spans the development of organic chemistry, materials science, and biotechnology. As dyes with high fluorescence efficiency and strong modification potential, Rhodamine synthesis methods have evolved from traditional acid-catalyzed condensation reactions to modern green chemistry approaches. This evolution meets laboratory needs for small amounts of high-purity products and drives industrial-scale production advances in cost control, environmental friendliness, and quality stability. Overall, Rhodamine synthesis pathways can be divided into two main categories:
- Classical condensation methods: Primarily involve the condensation of aromatic amines with phthalic acid derivatives to form the core structure, offering mature processes and controllable reaction conditions.
- Modern green methods: Employ novel catalysts, non-toxic solvents, or microwave-assisted techniques to improve yields and reduce environmental impact, aligning with sustainable development goals.
Researchers choose synthesis routes based on the target molecular structure, application scenario, and production requirements. For example, Rhodamine intended for bioconjugation requires high purity and specific functional groups, whereas Rhodamine for industrial materials or dyes prioritizes cost-effectiveness and scalability.
Common Synthetic Routes of Rhodamine Dyes
Rhodamine synthesis typically focuses on constructing the xanthene backbone and functional modification. Common routes include:
- Condensation reactions: The classic route involves condensing aromatic amines with phthalic anhydride or derivatives under acidic conditions, followed by cyclization to form the Rhodamine structure. This method is suitable for synthesizing basic Rhodamine dyes, such as Rhodamine B and Rhodamine 6G.
- Acylation and cyclization reactions: Aromatic compounds undergo acylation followed by cyclization under acidic conditions to form the Rhodamine core. This method allows structural control and the synthesis of various substituted derivatives.
- One-step and multi-step synthesis: Depending on the derivative's application, one-step synthesis can quickly yield the basic framework, while multi-step synthesis enables more complex functionalization, such as introducing hydrophilic groups, charged moieties, or reactive linkage sites.
- Functional modification routes: After forming the Rhodamine backbone, further functional groups can be introduced via amination, esterification, or click chemistry reactions, adapting the dye for bioconjugation, nanoprobe design, or sensor applications.
Advantages and Limitations of Each Synthesis Method
| Synthesis Method | Advantages | Limitations |
|---|---|---|
| Traditional Condensation Method | Mature process and easy operation; well-defined reaction conditions with good reproducibility; relatively high yield, suitable for large-scale production. | Requires strong acidic conditions, environmentally unfriendly; generates more by-products, making purification difficult. |
| Acylation-Cyclization Method | Flexible control of substituents; allows synthesis of diverse Rhodamine derivatives; suitable for functionalized structural design. | More complex reaction steps; relatively low overall yield; sensitive to reaction conditions. |
| Green Synthesis Approaches | Environmentally friendly and aligned with sustainable development; reduces use of toxic reagents; improves reaction efficiency and lowers environmental impact. | Requires novel catalysts or special equipment (e.g., microwave reactors); higher initial development cost. |
| Functionalization and Derivatization Strategies | Meets advanced application needs such as biolabeling and targeted imaging; enables molecular customization; broadens application scope. | More complex synthetic routes; requires fine separation and characterization; higher synthesis and purification costs. |
Key Challenges in Rhodamine Synthesis and Applications
Despite the great value of Rhodamine in research and industry, researchers often face a series of challenges during synthesis and application, which limit its wider adoption in larger-scale and more complex systems.
- Controlling Purity and By-Products in Rhodamine Synthesis: The synthesis of Rhodamine involves multiple chemical steps, and the presence of by-products and impurities can significantly affect its fluorescence performance and stability. Finding a balance between high yield and high purity is the key to optimizing the synthetic route. In particular, when scaling up to industrial production, the difficulty of purity control increases further.
- Solubility Issues in Aqueous and Organic Systems: Rhodamine derivatives show significant differences in solubility across solvents. Some derivatives exhibit poor solubility in aqueous systems, limiting their use in biological experiments; while in organic solvents, solubility is better but compatibility with biological systems must also be considered.
- Stability Problems Under Experimental Conditions: Rhodamine is prone to degradation or signal loss under the influence of pH, temperature, and light exposure during experiments. For example, some Rhodamine derivatives are easily photobleached under strong illumination, affecting the reproducibility and accuracy of experiments.
- Batch-to-Batch Variability in Scale-Up Production: In small-scale laboratory synthesis, batch variability of Rhodamine is relatively controllable. However, in industrial production, fluctuations in physicochemical properties between batches may occur, introducing uncertainty to research and applications. This issue is particularly critical for research and industrial uses requiring long-term stable supply.
Strategies to Overcome Difficulties in Rhodamine Synthesis
Although Rhodamine synthesis already has a well-established chemical foundation, challenges remain in industrial scale-up and translational applications. With increasing demand for fluorescent dyes in life sciences, diagnostics, and materials research, solving problems such as purity control, limited solubility, reduced stability, and poor scalability consistency has become the core task for synthesis and application optimization. Researchers and companies have developed various strategies to enhance the overall performance and reliability of Rhodamine.
Optimizing Synthetic Routes for High Yield and Consistency
Traditional Rhodamine synthesis usually involves reactions of aromatic aldehydes, phenols, and amines, during which by-products and isomers are easily formed. To improve yield and purity, researchers have introduced the following approaches:
- Green chemistry processes: Using aqueous reactions or ionic liquids instead of conventional organic solvents to reduce by-product formation and improve environmental sustainability; developing microwave-assisted synthesis to accelerate reaction rates and shorten reaction times.
- One-step and efficient catalysis: Employing Lewis acids or metal complexes as catalysts to reduce intermediate accumulation and improve selectivity; using heterogeneous catalysts for easy recovery and reuse, enhancing feasibility for industrial-scale synthesis.
- Automation and continuous flow reactions: Extending Rhodamine synthesis from batch reactions to continuous flow systems allows precise control of reaction conditions, reducing batch variability; continuous flow also improves heat transfer and quality control, making it suitable for large-scale preparation.
These improvements not only increase reaction yield and consistency but also effectively reduce purification costs, making Rhodamine commercialization more controllable.
Functionalization Approaches to Improve Solubility
A major issue for Rhodamine derivatives in applications is limited solubility. Common strategies to address this include:
- Hydrophilic group modification: Introducing sulfonic acid, carboxyl, or amino groups into the molecular structure to enhance aqueous solubility; using polyethylene glycol (PEG) chains to improve molecular dispersion stability in biological environments.
- Amphiphilic molecular design: Linking both hydrophobic and hydrophilic groups to Rhodamine to create amphiphilic probes that maintain fluorescence while achieving good solubility in complex systems.
- Carrier systems: Employing nanoparticles, liposomes, or polymer micelles to encapsulate Rhodamine, improving dispersion in aqueous environments and reducing nonspecific interactions with biomolecules.
Through these functionalization strategies, researchers can obtain Rhodamine probes that retain fluorescence while achieving excellent biocompatibility.
Stabilization Techniques for Long-Term Storage and Use
Rhodamine may suffer from photobleaching, thermal degradation, or chemical degradation in applications. To address these stability challenges, researchers have proposed multiple solutions:
- Molecular design: Introducing electron-donating or electron-withdrawing groups into the Rhodamine backbone to tune energy levels and reduce photosensitivity; designing rigid derivatives to minimize intramolecular rotation and non-radiative decay, thereby improving photostability.
- Optimized storage and handling conditions: Storing Rhodamine under low temperature, dark conditions, and inert atmosphere (such as nitrogen or argon); using lyophilization to prepare solid powder forms for extended shelf life.
- External protection and encapsulation: Using polymer coatings, protein complexes, or nanocarriers to shield Rhodamine from environmental impact; applying antioxidants (e.g., ascorbic acid, glutathione) in biological applications to reduce damage from reactive oxygen species.
The combination of these stabilization strategies can significantly extend Rhodamine's usable lifetime and ensure consistent performance during long-term experiments or storage and transport.
Quality Considerations in Rhodamine Synthesis
High-quality Rhodamine is essential for both research and industrial applications. The purity, stability, and optical performance of the dye directly determine its effectiveness in biological imaging, fluorescent labeling, and nanotechnology. Whether in laboratory research or industrial production, ensuring the consistency and reliability of Rhodamine products is a central goal of synthesis process design and quality control. Through rigorous quality management and process optimization, Rhodamine dyes deliver stable and reliable performance in high-sensitivity fluorescence detection, precise biomolecular labeling, and large-scale industrial applications, providing a solid foundation for scientific and industrial work.
Purity, Stability, and Spectral Characteristics
- Purity Control: High-purity Rhodamine is fundamental for obtaining reliable experimental results. Techniques such as high-performance liquid chromatography (HPLC), mass spectrometry (MS), and nuclear magnetic resonance (NMR) allow precise detection of by-products and unreacted precursors, ensuring dye purity meets research or industrial requirements. High purity not only enhances fluorescence signal intensity and stability but also reduces nonspecific binding and background interference.
- Spectral Characteristics: The absorption and emission wavelengths of Rhodamine should remain stable during production and storage to avoid experimental variability due to batch differences. Strict control of chemical modification and solvent conditions ensures consistent optical properties, making the dye suitable for multicolor fluorescence imaging, FRET analysis, and other precise measurements.
- Long-Term Stability: Rhodamine must remain stable under storage and transport conditions without degradation or self-quenching. Typically, storage in dry, dark, and low-temperature conditions, sometimes with stabilizers, effectively prolongs dye lifespan and ensures reliability in both research and industrial applications.
Scale-Up Considerations for Industrial and Research Use
- Laboratory to Industrial Scale-Up: When scaling from small-scale laboratory synthesis to industrial production, attention must be paid to controllability of reaction conditions, including solvent volume, temperature, heat management, and catalyst and reagent recovery. Proper design of reactors and process flows can improve yield, reduce costs, and maintain dye optical performance and purity.
- Process Validation: Strict process validation is essential during scale-up to ensure that different batches of Rhodamine maintain high consistency in purity, spectral characteristics, and functional capacity. This is critical for experimental reproducibility and standardized industrial production.
- Regulatory Compliance: Research-grade and pharmaceutical-grade Rhodamine products must comply with relevant standards, such as ISO, GMP, or GLP. This includes production environment control, raw material traceability, quality testing, and documentation to ensure the product meets scientific requirements and rigorous preclinical or drug development standards.
How to Enhance Rhodamine Performance in Biological Applications?
The derivatization of Rhodamine is a key step for its applications in biology, chemical sensing, and materials science. Functional modification enables Rhodamine dyes to gain new binding properties and water solubility, as well as achieve targeted recognition. These modifications are particularly important in modern fluorescence technologies, especially for molecular targeting, protein conjugation, and nanomaterial applications, enabling site-specific labeling and high-selectivity molecular recognition.
Introducing Functional Groups for Bioconjugation
To achieve bioconjugation, reactive functional groups are usually introduced into the Rhodamine backbone to covalently bind with biomolecules or nanomaterials. Common functional groups include:
- Carboxyl (-COOH): Carboxylated Rhodamine can be activated by EDC/NHS to form stable amide bonds with amino groups on proteins or peptides. This method is simple and commonly used for protein labeling and fluorescent probe preparation.
- Isothiocyanate (-NCS): Highly reactive, can form stable thiourea bonds with primary amines on proteins or peptides, widely used for labeling antibodies, peptides, and other biomolecules.
- Maleimide: Selectively reacts with thiol groups (-SH) to label cysteine residues on proteins or thiol-modified oligonucleotides, enabling precise and controllable conjugation while reducing non-specific binding.
- Hydrophilic groups: Introducing hydroxyl (-OH), sulfonic (-SO₃H), or phosphate (-PO₄H₂) groups significantly enhances Rhodamine solubility in aqueous and biological systems.
- PEGylation: Attaching polyethylene glycol (PEG) chains forms a flexible hydrophilic barrier on the Rhodamine molecule, improving solubility and stability. PEG modification also reduces non-specific binding to off-target biomolecules and prolongs in vivo circulation time.
Strategies for Targeted Fluorescent Labeling
Rhodamine derivatization is not only a chemical modification but also a core strategy for directional biological labeling and signal amplification. Common application strategies include:
- Antibody labeling: Reaction of carboxyl or isothiocyanate groups with amino groups on antibodies enables highly specific fluorescent labeling for immunohistochemistry, flow cytometry, and live imaging. This approach preserves antibody recognition while providing stable Rhodamine fluorescence.
- Peptide/protein probe development: Functionalized Rhodamine can be conjugated to peptides or proteins to create highly specific molecular probes. By precisely designing binding sites, these probes can specifically recognize cell receptors, enzyme activity, or signaling molecules, widely used in cell metabolism studies and drug screening.
- Nanoparticle modification: Functionalized Rhodamine molecules can be attached to nanoparticle surfaces such as gold nanoparticles, quantum dots, or liposomes. This strategy enables signal enhancement, targeted delivery, and theranostic integration, playing a key role in bioimaging, targeted drug delivery, and optical sensor development.
Imaging and Detection in Cellular and Molecular Research
Rhodamine and its derivatives also play a central role in cellular and molecular imaging and detection:
- Cell imaging: Targeted modifications of Rhodamine enable specific labeling of mitochondria, cell membranes, or nucleic acids, facilitating organelle localization, dynamic observation, and live-cell tracking. Its high photostability and controllable fluorescence intensity allow long-term imaging.
- Molecular probe development: Functionalized Rhodamine can bind to specific targets, such as enzyme active sites, receptors, or small molecule ligands, producing real-time detection probes. These probes exhibit high sensitivity and specificity in drug screening, signaling pathway research, and disease mechanism studies.
- Nanobiotechnology: Rhodamine can be combined with nanocarriers such as gold nanoparticles, quantum dots, or liposomes for signal enhancement, targeted delivery, and integrated theranostic applications. In bioimaging and drug delivery, this nanoparticle labeling strategy not only increases detection sensitivity but also allows monitoring of drug distribution and release through fluorescence signals.
BOC Sciences' Expertise in Custom Rhodamine Synthesis
With a well-established organic synthesis platform and extensive expertise in fluorescent probe development, BOC Sciences provides custom synthesis services for high-purity and high-stability rhodamine and its derivatives to global research and industry clients. Tailoring each project to specific research goals, we design flexible synthetic routes, fine-tune molecular scaffolds, substituents, and functional modifications to ensure optimal optical performance, solubility, and stability for experimental applications.
Rhodamine Structural Diversification
- Comprehensive Core Structures: Including Rhodamine B, Rhodamine 6G, Rhodamine 123, and other derivatives, covering a wide range of excitation/emission wavelengths.
- Spectral Tuning Design: Modification with different substituents to adjust emission maxima, enabling controllable emission from green to red.
- Rigid Structure Optimization: Development of cyclic or fused-ring derivatives to minimize non-radiative relaxation and enhance quantum yield.
- Amphiphilic Molecular Structures: Balanced design that maintains fluorescence properties while improving water solubility and membrane permeability for biological systems.
Rhodamine Functionalization Strategies
- Hydrophilicity Enhancement: Introduction of carboxyl, sulfonate, or PEG groups to improve aqueous solubility and biocompatibility.
- Reactive Linker Integration: Availability of NHS ester, maleimide, azide, and other reactive moieties for efficient conjugation with proteins, peptides, or nucleic acids.
- Targeted Modification: Conjugation with biomolecules such as sugars or peptides to endow rhodamine probes with specific cellular or protein recognition.
- Stability Enhancement: Incorporation of anti-photobleaching groups or encapsulation in nanocarriers to extend probe lifetime in imaging applications.
Custom Rhodamine Synthesis
- Tailored Synthetic Route Design: Development of efficient, low-byproduct synthetic pathways aligned with the client's research objectives to ensure high yield and purity.
- Modular Functionalization: Flexible introduction of substituents or functional groups to achieve spectral tuning, improved solubility, or bio-conjugation capabilities.
- Scalable Production: Support for projects ranging from milligram-scale R&D batches to kilogram-scale manufacturing, meeting both research and industrial demands.
- Rigorous Quality Control and Characterization: Comprehensive analysis using HPLC, NMR, MS, and spectroscopic methods to guarantee batch-to-batch consistency and reliability.
Rhodamine Bulk Supply
- Extensive Inventory: A wide range of common rhodamine derivatives available in stock for immediate delivery.
- Flexible Specifications: Supply options from milligram-scale research quantities to multi-gram and hundred-gram industrial-scale batches.
- Batch Consistency: Strict quality control processes ensure reproducibility in purity, optical properties, and stability across different lots.
- Global Supply Chain Support: Compliance with international shipping and regulatory standards to deliver efficient and reliable supply worldwide.
Do You Need A Consultation?
BOC Sciences integrates cutting-edge fluorescence technologies to accelerate your research, driving next-generation solutions for drug discovery and diagnostics.
Transform Your Studies with Cutting-Edge Fluorescent Products
| Catalog | Name | CAS | Inquiry |
|---|---|---|---|
| A16-0170 | Rhodamine-123 | 62669-70-9 | Bulk Inquiry |
| A14-0036 | Rhodamine B hydrazide | 74317-53-6 | Bulk Inquiry |
| F05-0031 | 6-Carboxy-X-rhodamine | 194785-18-7 | Bulk Inquiry |
| A17-0069 | Rhodamine 590 Chloride | 3068-39-1 | Bulk Inquiry |
| A17-0106 | Rhodamine 19 Perchlorate | 62669-66-3 | Bulk Inquiry |
| A16-0014 | Sulforhodamine 101 | 60311-02-6 | Bulk Inquiry |
| A17-0061 | Rhodamine 610 Perchlorate | 23857-51-4 | Bulk Inquiry |
| A16-0093 | Rhodamine 6G | 989-38-8 | Bulk Inquiry |
| A01-0005 | Rhodamine B | 81-88-9 | Bulk Inquiry |
| A17-0107 | Rhodamine 640 Perchlorate | 72102-91-1 | Bulk Inquiry |
High-Performance Fluorescent Tools for Your Research
- BODIPY Bright, stable dyes used in bioimaging and molecular labeling.
- Coumarin Blue-emitting dyes for chemical sensing and fluorescence studies.
- Cyanine Versatile dyes used in biosensing and nucleic acid detection.
- Fluorescent Protein Used for live-cell imaging and real-time biosensing.
More About Rhodamine Dyes
Online Inquiry

