How to Effectively Stain Lipid Droplets Using BODIPY Dyes: A Practical Guide for Researchers
Lipid droplets (LDs), as crucial intracellular centers for energy storage and signal regulation, play key roles in metabolism, cellular stress responses, and the development of various diseases. Accurate visualization of lipid droplets not only helps reveal their biological functions but also provides critical insights for drug screening and mechanistic studies. BODIPY dyes, with their high lipophilicity, strong selectivity, and stable fluorescence, have become the preferred tools for lipid droplet staining and imaging. This guide offers researchers a practical approach to BODIPY-based lipid droplet imaging, covering dye selection, experimental procedures, imaging techniques, and troubleshooting tips, aiming to help scientists overcome experimental challenges and obtain high-quality, reliable lipid droplet imaging data.
Understanding Lipid Droplet Imaging in Modern Research
With the continuous advancement of cell biology and metabolism research, lipid droplets have emerged as important subjects for studying energy metabolism, signal transduction, and disease development. Beyond serving as storage depots for neutral lipids, lipid droplets are closely associated with obesity, diabetes, fatty liver, cardiovascular diseases, and even tumor metabolism. Therefore, precise imaging and quantitative analysis of lipid droplets have become essential tools in life sciences research.
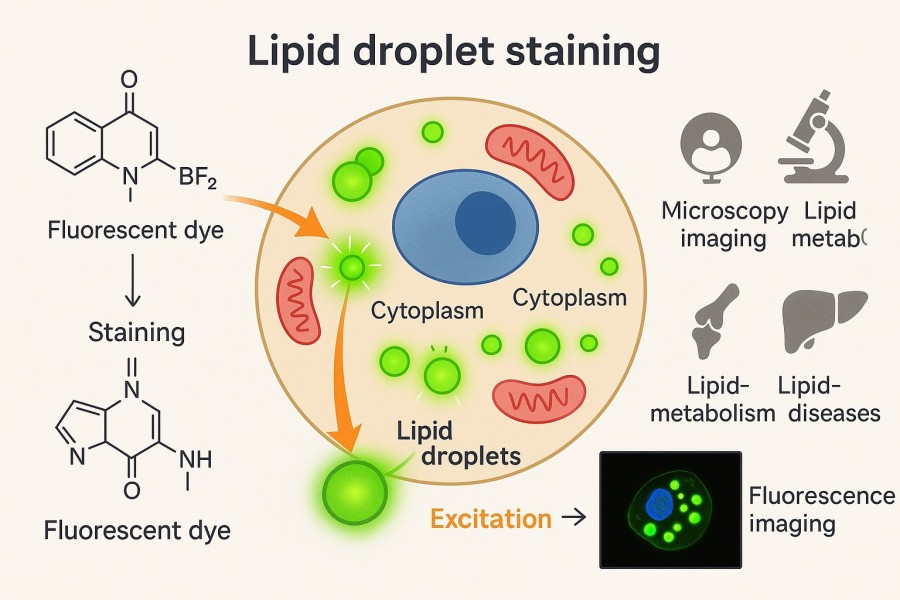 Fig. 1. Lipid droplet staining (BOC Sciences Authorized).
Fig. 1. Lipid droplet staining (BOC Sciences Authorized).
Biological Roles of Lipid Droplets
Lipid droplets are highly dynamic organelles whose biological functions extend far beyond mere energy storage. They have become versatile players in cell function research, and accurate imaging of their morphology and dynamics is fundamental to understanding these roles.
- Regulation of energy metabolism: Lipid droplets are rich in triacylglycerols (TAGs) and cholesteryl esters (CEs), serving as the primary energy reservoir of the cell. When energy is scarce, lipids within lipid droplets can be broken down via lipolysis, releasing free fatty acids to support mitochondrial oxidation and maintain cellular metabolic demands.
- Regulation of signaling molecules: Lipid droplets act not only as metabolic storage but also as sites for the synthesis and regulation of lipid signaling molecules. For example, arachidonic acid and other lipid mediators can be released from lipid droplets, further regulating inflammation, cell growth, and immune responses.
- Cellular protection: Under oxidative stress or lipotoxic conditions, lipid droplets can sequester harmful lipids, preventing damage to cellular membranes and organelles. This "detoxification" function is particularly important in pathological conditions such as liver disease and diabetes.
- Protein quality control: Increasing evidence shows that lipid droplets play a role in protein degradation and homeostasis. Misfolded or damaged proteins can associate with lipid droplets and be subsequently cleared via autophagy or proteasomal pathways.
Importance of Lipid Droplet Visualization in Cell Biology
The importance of lipid droplet imaging can be summarized in several key aspects:
- Revealing cellular metabolic states: The number and size of lipid droplets serve as direct indicators of cellular energy metabolism. For instance, abnormal lipid droplet accumulation in hepatocytes is often associated with fatty liver disease, whereas a reduction in lipid droplet numbers may reflect heightened metabolism or stress-induced damage.
- Exploring organelle interactions: Lipid droplets do not exist in isolation; they are closely associated with mitochondria, endoplasmic reticulum, lysosomes, and peroxisomes. The spatial proximity and dynamic interactions between lipid droplets and these organelles determine lipid synthesis, transport, and breakdown efficiency. High-resolution imaging allows researchers to uncover the crucial roles of lipid droplets in organelle crosstalk.
- Drug screening and functional validation: In drug development, lipid droplet imaging is a vital method to assess how candidate compounds affect lipid metabolism. Compounds that modulate lipid metabolism often induce changes in lipid droplet number or distribution. Quantitative imaging enables rapid evaluation of drug effects.
- Disease mechanism studies: Lipid droplets play important roles in obesity, diabetes, atherosclerosis, and cancer. Precise visualization of lipid droplets helps reveal the molecular mechanisms underlying disease progression, facilitating improvements in diagnostic and therapeutic strategies.
Key Imaging Techniques Used for Lipid Droplets
Modern lipid droplet research relies on multiple imaging techniques, each with its advantages and limitations. Among these, fluorescence microscopy and flow cytometry are the most commonly used methods, with the choice of high-performance dyes such as the BODIPY series being critical for optimal imaging outcomes.
- Fluorescence Microscopy: Using lipid droplet-specific fluorescent dyes (e.g., BODIPY, Nile Red), researchers can quickly and visually observe lipid droplet distribution and number. This method is cost-effective and straightforward, making it one of the most commonly used techniques, though it has certain limitations in resolution and fine structural detail.
- Confocal Microscopy: By scanning layer by layer, confocal microscopy provides three-dimensional spatial information of lipid droplets within cells and effectively reduces background interference. This approach is particularly useful for studying the spatial relationships and dynamics of lipid droplets with mitochondria, ER, and other organelles.
- Flow Cytometry: Combined with lipid droplet staining, flow cytometry allows quantitative analysis of lipid droplet content in thousands of cells, offering high-throughput and statistical advantages. This technique is ideal for drug screening and population-level studies of lipid metabolism.
- Super-Resolution Microscopy: Techniques such as STED, SIM, and STORM overcome the diffraction limit of conventional optical microscopy, clearly revealing fine structures of lipid droplet-associated proteins and lipids. These methods are essential for mechanistic studies and high-resolution imaging needs.
- Electron Microscopy (EM): EM provides ultra-high-resolution images of lipid droplets, enabling direct observation of their ultrastructural relationships with other organelles. Although sample preparation is complex and dynamic processes cannot be captured, EM remains indispensable for structural studies.
Why Choose BODIPY Dyes for Lipid Droplet Staining?
Lipid droplet imaging demands high selectivity, sensitivity, and signal stability. Among numerous lipid droplet dyes, BODIPY dyes stand out due to their unique chemical and optical properties, making them the preferred choice for lipid droplet staining. Compared to traditional dyes, BODIPY provides strong fluorescence signals and maintains reliable imaging performance under diverse experimental conditions. The advantages of BODIPY dyes in lipid droplet staining can be summarized in three aspects:
Advantages of BODIPY over Nile Red and Other Dyes
Compared with traditional dyes such as Nile Red, BODIPY dyes exhibit narrower emission peaks and higher photostability. This reduces signal overlap in multi-label experiments and maintains clarity during prolonged imaging. In addition, BODIPY's strong fluorescence signal ensures clear images even at low staining concentrations, significantly improving sensitivity and reliability. In contrast, Nile Red is more sensitive to environmental polarity, exhibits higher background fluorescence, and is prone to photobleaching during extended observation, limiting its use in high-precision experiments.
Lipophilicity and Selectivity for Neutral Lipids
BODIPY dyes are naturally lipophilic and specifically incorporate into the neutral lipid core of lipid droplets, such as triacylglycerols and cholesteryl esters. This high lipophilicity ensures precise intracellular localization while minimizing non-specific staining. Researchers can use BODIPY dyes to clearly observe lipid droplet number, size, and distribution, enabling analysis of cellular metabolic status or drug intervention effects and providing reliable data for metabolic studies.
Compatibility with Fluorescence Microscopy and Flow Cytometry
The spectral properties of BODIPY dyes are highly compatible with most fluorescence microscopes and flow cytometry filter sets, allowing researchers to directly use existing instruments for imaging and quantitative analysis. Their broad excitation/emission ranges cover multiple green and red fluorescence channels, supporting multiplexed imaging experiments. BODIPY dyes are stable in both live and fixed cells, enabling dynamic observation and quantitative studies, greatly facilitating lipid droplet research.
BODIPY Staining Services by BOC Sciences
| Solutions | Capabilities |
|---|---|
| Lipid Staining | Enables high-contrast visualization of lipid structures with customizable BODIPY probes and optimized fluorescence stability. |
| Cell Staining | Supports accurate and multiplexed cell labeling with dyes compatible across live and fixed cell imaging. |
| DNA Staining | Provides sensitive and specific detection of nucleic acids with minimal background interference. |
| RNA Staining | Facilitates reliable RNA visualization using spectrally optimized BODIPY variants for high-resolution imaging. |
| Protein Staining | Achieves precise labeling of proteins and antibodies through functionalized BODIPY conjugates and validated conjugation protocols. |
Step-by-Step Protocol for Staining Lipid Droplets with BODIPY
To help researchers successfully perform lipid droplet staining experiments, here is an optimized step-by-step guide covering sample preparation, dye usage, experimental conditions, imaging, and data analysis. By following these procedures, researchers can achieve high signal-to-noise ratio images with minimal background interference.
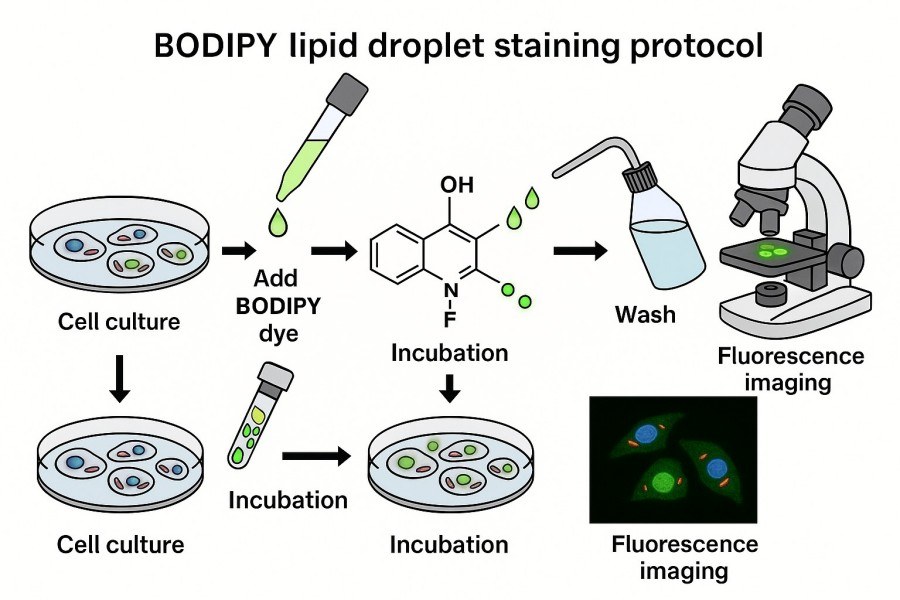 Fig. 2. BODIPY lipid droplet staining protocol (BOC Sciences Authorized).
Fig. 2. BODIPY lipid droplet staining protocol (BOC Sciences Authorized).
Sample Preparation (Live and Fixed Cell Imaging with BODIPY Dyes)
- Live cell staining: BODIPY dyes are suitable for direct staining of live cells, allowing real-time observation of lipid droplet dynamics. Ensure cells are healthy prior to staining to avoid abnormal lipid droplet formation caused by over-confluence or stress. It is recommended to gently wash cells with a mild buffer (e.g., HBSS) before staining to remove residual culture media and reduce background signal.
- Fixed cell staining: For fixed cells, mild fixation using low-concentration paraformaldehyde (2–4%) for 10–15 minutes is recommended to preserve lipid droplet structure. After fixation, wash cells multiple times with PBS or other suitable buffers to remove residual fixative while preventing droplet deformation or shrinkage. Fixed cell staining is ideal for high-resolution imaging and combined immunofluorescence experiments.
Optimal Dye Concentration and Incubation Time
For optimal signal, select dye concentration and staining duration according to the experiment type and cell line.
- Dye concentration: Recommended working range is 0.5–2 µM. Lower concentrations reduce non-specific staining, while higher concentrations may lead to dye aggregation or increased background.
- Staining time: Typically 15–30 minutes; live cell staining favors shorter durations to minimize cytotoxicity, whereas fixed cell staining can be slightly extended to ensure complete labeling of lipid droplets.
A small-scale pilot experiment is recommended to optimize concentration and time, balancing signal intensity with specificity.
Suggested Buffers, Solvents, and Controls
Solvents and buffers:
- Solvents: DMSO or ethanol can dissolve BODIPY dyes, but should be diluted to a final concentration<0.1% to avoid cytotoxicity.
- Buffers: PBS or HBSS can be used for washing before and after staining, maintaining cell permeability and pH stability.
Experimental controls:
- Blank control: Unstained cells to assess intrinsic cellular fluorescence background.
- Solvent control: Cells treated with solvent only to evaluate the effect of the solvent on signal and cell condition.
- Proper control setup helps researchers accurately assess staining efficiency and signal specificity.
Imaging and Data Analysis Tips
- Imaging optimization: Use low light intensity and short exposure times to reduce photobleaching effects. For live cell experiments, consider a temperature-controlled microscope or an imaging environment with CO₂ regulation to maintain normal cellular metabolism.
- Data analysis: Image analysis software (e.g., ImageJ/Fiji) is recommended to quantify lipid droplet number, area, and fluorescence intensity. Thresholding and batch processing of multiple images improve reliability and reproducibility. Comparing experimental and control groups allows effective evaluation of drug treatments or gene interventions on lipid droplet formation.
- Additional tips: Image samples immediately after staining to minimize fluorescence decay. For multi-channel imaging, choose BODIPY variants with non-overlapping spectra to avoid signal interference.
Troubleshooting Common Issues in BODIPY Lipid Staining
Although BODIPY dyes perform excellently in lipid droplet staining, common issues may still arise, such as weak signal, high background, dye aggregation, or photobleaching. Detailed explanations and solutions for these issues are provided below to help optimize experimental conditions and improve imaging quality.
Weak Fluorescence Signal or High Background
This is a frequent problem, often caused by insufficient dye concentration, short staining duration, or poor cell condition. Residual culture media or fixatives can also interfere with fluorescence. Solutions include adjusting BODIPY concentration to the recommended range (0.5–2 µM), slightly extending staining time, using freshly prepared dye solutions, and ensuring thorough cell washing before and after staining. To reduce high background, optimize washing steps or select appropriate buffers (PBS/HBSS) to minimize non-specific fluorescence.
Dye Aggregation or Non-Specific Binding
Aggregation or non-specific binding can lead to uneven signals and local background enhancement, affecting lipid droplet quantification and localization. Causes may include excessive dye concentration, improper solvent usage, or prolonged dye storage. Solutions: use lower dye concentrations, dilute solvents to<0.1% final concentration to minimize cytotoxicity, prepare fresh dye solutions and mix thoroughly before adding to samples. Extending washing steps can remove free or non-specifically bound dye, yielding clearer lipid droplet signals.
Photobleaching or Signal Instability During Imaging
Photobleaching and unstable signals affect time-lapse imaging and quantitative analysis, particularly in live cell dynamic studies. Main causes include high excitation intensity, long exposure times, or unstable imaging temperature. Solutions: use low light intensity, shorten exposure time or interval shooting; apply anti-fade mounting media; maintain stable temperature and CO₂ during live cell imaging to reduce metabolic stress on fluorescence. Selecting photostable BODIPY variants can also effectively minimize photobleaching effects.
Choosing the Right BODIPY Dyes for Lipid Droplet Imaging
Selecting the appropriate BODIPY dye is critical for lipid droplet imaging, as different BODIPY variants vary in optical performance, fluorescence stability, and cellular compatibility. A well-chosen dye can provide clear images of lipid droplets, minimize background interference and photobleaching, and support multiplexed fluorescence experiments. The following sections detail considerations regarding dye variants, spectral properties, and suitability for different cell types.
Comparison of BODIPY 493/503, BODIPY 505/515, and Other Variants
BODIPY 493/503 is a classic lipid droplet dye with medium excitation wavelength and green emission, compatible with standard filters on most fluorescence microscopes. It demonstrates high selectivity and stability in both live and fixed cells, making it ideal for observing lipid droplet number and volume changes. In contrast, BODIPY 505/515 emits slightly yellow-green light, suitable for combination with other green or blue fluorescence channels, enabling multiplexed experiments without signal interference. Other BODIPY variants, such as red or far-red series, can be used when imaging alongside green or blue labels, offering strong photostability and cellular compatibility. By selecting the appropriate variant according to experimental needs, researchers can maximize signal intensity and imaging clarity.
Excitation/Emission Spectra and Filter Set Compatibility
When choosing a BODIPY dye, it is important to consider the match between its excitation/emission spectra and the microscope filter sets. BODIPY dyes typically have narrow emission peaks, reducing spectral overlap and facilitating multi-channel imaging. During experiment design, select variants compatible with existing filters to achieve optimal signal-to-noise ratio. For example, in multiplexed experiments, green BODIPY variants can be combined with red or far-red fluorescent proteins to achieve clear separation of signals, enabling effective analysis of spatial relationships between lipid droplets and other organelles.
Considerations for Live vs. Fixed Cell Imaging
BODIPY dyes perform slightly differently in live versus fixed cell imaging. For live cell experiments, choose low-toxicity, highly photostable variants to ensure normal metabolism and dynamic observation of lipid droplets, while controlling dye concentration and staining time to avoid cellular stress. For fixed cells, dye permeability and resistance to fixatives are especially important; variants stable under paraformaldehyde or ethanol fixation are recommended. Staining time can be extended in fixed cells to enhance signal, and optimized washing steps can reduce background interference. Selecting appropriate dyes and staining conditions according to experiment type can significantly improve imaging success and reproducibility.
Get Custom Support and High-Quality BODIPY Dyes from Our Experts
In the field of BODIPY fluorescent dyes, BOC Sciences leverages extensive expertise and a robust technical platform to provide high-quality, customized services for researchers and companies. Whether it involves selecting base dyes, functional modifications, or application optimization, our expert team offers professional guidance and personalized support to help clients achieve efficient, precise experimental design and successful outcomes.
Custom Synthesis of BODIPY Lipid Probes
- Provide custom lipid probes with specific spectral and structural properties according to client requirements.
- Support functional modifications, such as conjugation to biomolecules or membrane-targeting modifications.
- Offer flexible synthesis capabilities from small-scale laboratory samples to pilot-scale production.
- Ensure compound structural accuracy and batch-to-batch consistency to meet research and industrial application needs.
BODIPY Conjugation Support Services
- Provide efficient conjugation strategies for BODIPY dyes with proteins, antibodies, oligonucleotides, and other biomolecules.
- Support multiple chemical conjugation methods, including active esters, maleimides, and click chemistry.
- Assist in optimizing conjugation conditions to ensure high labeling efficiency without affecting biomolecule function.
- Offer purity and functional validation of conjugates to ensure reliability in downstream experiments.
Bulk Supply and Purity Verification
- Supply high-purity BODIPY products in large quantities to support research and industrial projects.
- Strictly perform purity testing and quality control using HPLC, NMR, and other analytical methods.
- Provide purity and performance reports to deliver reliable data support for client experiments.
- Offer flexible inventory management and supply solutions to respond quickly to customer needs.
Technical Support for Assay Optimization and Troubleshooting
- Provide guidance on experimental design, method optimization, and strategies to enhance fluorescence signals.
- Assist in addressing common issues such as weak fluorescence, background interference, or dye stability.
- Offer tailored technical advice for cell imaging, membrane studies, and molecular interaction experiments.
- Senior experts provide online support to ensure efficient and reliable execution of client experiments.
Do You Need A Consultation?
BOC Sciences integrates cutting-edge fluorescence technologies to accelerate your research, driving next-generation solutions for drug discovery and diagnostics.
Transform Your Studies with Cutting-Edge Fluorescent Products
| Catalog | Name | CAS | Inquiry |
|---|---|---|---|
| F01-0166 | BODIPY 493/503 NHS Ester | 216961-98-7 | Bulk Inquiry |
| F01-0161 | BODIPY 558/568 C12 | 158757-84-7 | Bulk Inquiry |
| F01-0045 | BODIPY 505/515 | 21658-70-8 | Bulk Inquiry |
| F01-0044 | BODIPY-Cholesterol | 878557-19-8 | Bulk Inquiry |
| R12-0001 | BODIPY 493/503 | 121207-31-6 | Bulk Inquiry |
| F01-0257 | C11 BODIPY 581/591 | 217075-36-0 | Bulk Inquiry |
| F01-0259 | BODIPY 16 | 2654002-78-3 | Bulk Inquiry |
| F01-0251 | BODIPY 576/589 | 150173-78-7 | Bulk Inquiry |
| F01-0254 | BODIPY 493/503 carboxylic acid | 216961-95-4 | Bulk Inquiry |
| F01-0188 | BODIPY 576/589 SE | 201998-61-0 | Bulk Inquiry |
High-Performance Fluorescent Tools for Your Research
- Alexa Fluor Bright, stable dyes for sensitive biosensing applications.
- Cyanine Versatile dyes used in biosensing and nucleic acid detection.
- Rhodamine Strong fluorescence, commonly used in protein and DNA sensing.
- Fluorescent Protein Used for live-cell imaging and real-time biosensing.
More About BODIPY Dyes
Online Inquiry

