Complete Guide to BODIPY Staining for Reliable Research: Principle and Protocol
BODIPY staining is a widely applied fluorescent labeling method in cell and tissue research, highly valued by researchers for its high sensitivity and stable signal. With the unique chemical structure and excellent optical properties of BODIPY dyes, scientists can directly visualize lipid droplet distribution, organelle function, and molecular dynamics. This technique not only plays an important role in fundamental cell biology and metabolic mechanism studies but is also extensively used in drug screening, disease model construction, and live-cell imaging. To achieve reliable experimental results, researchers need a deep understanding of the bodipy staining principle, master the bodipy staining protocol under different experimental systems, and flexibly optimize conditions based on sample characteristics.
What is BODIPY Staining?
BODIPY staining is a technique that uses BODIPY fluorescent dyes to label biological samples, widely applied in cell, tissue, and microbial studies. Through BODIPY staining, researchers can directly observe lipid distribution, organelle structures, and dynamic changes of specific biomolecules, providing reliable data for cell biology, metabolic research, and drug screening.
Overview of BODIPY Fluorescent Dyes
BODIPY fluorescent dyes are a class of boron-containing, highly stable organic molecules with unique optical properties and chemical stability, giving them great application value in fluorescence labeling. Compared with conventional fluorescent dyes, BODIPY dyes feature narrow emission peaks, high quantum yield, and low photobleaching, demonstrating outstanding stability in long-term imaging experiments. In addition, different BODIPY derivatives can be functionally modified according to experimental needs, such as conjugation with lipids, proteins, or nucleic acids, enabling multichannel fluorescence imaging and quantitative analysis. These characteristics make BODIPY dyes widely adopted in experiments such as lipid droplet staining and adipocyte staining.
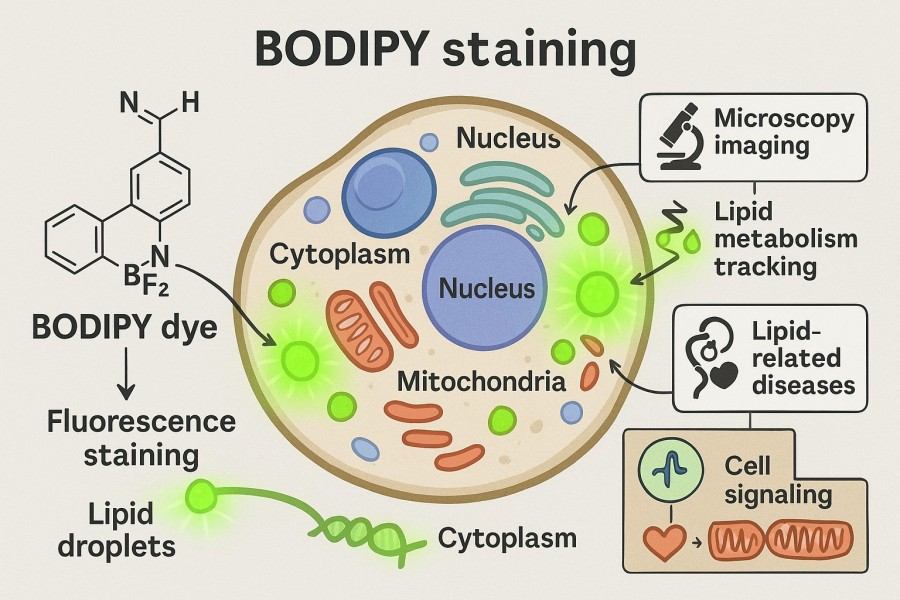 Fig. 1. BODIPY staining protocol (BOC Sciences Authorized).
Fig. 1. BODIPY staining protocol (BOC Sciences Authorized).
Unique Properties of BODIPY Dyes for Staining Applications
BODIPY dyes exhibit several unique advantages in biological staining applications:
- High photostability: Signals remain stable even under prolonged excitation, supporting time-lapse imaging and dynamic observation.
- Narrow emission spectra: Facilitate multicolor fluorescence imaging, minimize signal overlap, and enable simultaneous multi-target analysis.
- Low background interference: Greatly improves signal-to-noise ratio, making quantitative analysis more reliable and suitable for high-precision experiments.
- Flexible chemical modification: BODIPY dyes can be structurally modified to become hydrophilic or hydrophobic, allowing customized staining for lipid droplets, membranes, and organelles.
Thanks to these superior properties, BODIPY staining has become an essential tool for studying lipid metabolism, membrane structures, cell signaling, and microbial research, and is also the preferred dye for applications such as fixed cell staining.
What is the Principle of BODIPY Staining?
The principle of BODIPY staining mainly relies on its unique chemical structure, fluorescence mechanism, and specific interactions with biological targets. This staining technique not only provides bright and stable fluorescence signals but also enables accurate labeling of lipid droplets, adipocytes, membrane proteins, microorganisms, and tissue sections. By understanding the structural features of BODIPY dyes and their biological interaction mechanisms, researchers can more effectively design bodipy staining protocols and overcome common issues such as weak signals, high background, or poor reproducibility.
Chemical Structure and Fluorescence Mechanism
The core of a BODIPY molecule is formed by two pyrrole rings and a boron atom, creating a conjugated system. Its highly conjugated π-electron structure is the key source of fluorescence properties. When samples are excited with appropriate wavelengths, electrons in the BODIPY molecule transition from the ground state to the excited state. Subsequently, electrons return to the ground state via controlled radiation, releasing detectable fluorescence at specific wavelengths. This efficient fluorescence emission mechanism not only ensures bright and stable signals but also maintains excellent performance in various experimental conditions such as fixed cell staining and tissue staining. The narrow emission spectra and high quantum yield of BODIPY dyes make them particularly effective in multichannel fluorescence imaging, significantly reducing signal interference and improving reproducibility. Furthermore, different BODIPY derivatives can be chemically modified to adjust spectra or hydrophilic/hydrophobic properties, thereby meeting diverse experimental needs, including bodipy staining lipid droplets and lipid oxidation studies with C11-BODIPY staining.
Interaction of BODIPY with Biological Targets
BODIPY dyes can interact with biomolecules via hydrophobic interactions, electrostatic forces, or covalent bonding. For example, BODIPY-lipid derivatives specifically bind to lipid droplets, C11-BODIPY is used in oxidative stress studies, while BODIPY-protein conjugates can form stable complexes with protein amino acid residues. By understanding these binding mechanisms, researchers can optimize dye concentration, incubation time, and buffer conditions, thus improving bodipy staining performance and achieving high signal-to-noise ratio with minimal background interference.
BODIPY Staining Services by BOC Sciences
| Solutions | Capabilities |
|---|---|
| Lipid Staining | High-purity BODIPY dyes and tailored derivatives for precise lipid droplet visualization and lipid metabolism studies. |
| Cell Staining | Versatile BODIPY probes for reliable staining of live and fixed cells, with customizable modifications to match diverse research needs. |
| DNA Staining | Functionalized BODIPY derivatives for sensitive and accurate DNA detection with stable fluorescence performance. |
| RNA Staining | Customized BODIPY-based probes for selective RNA labeling, enabling advanced transcriptomic research. |
| Protein Staining | BODIPY conjugation options for specific protein labeling, ensuring stable fluorescence for protein tracking and functional studies. |
How to Make BODIPY Staining Solution?
Preparing high-quality BODIPY staining solutions is a key step for successful experiments. Factors such as dye concentration, solubility, storage conditions, and mixing methods directly affect staining performance, signal intensity, and reproducibility. When preparing staining solutions, researchers should select suitable solvents and working concentrations based on sample type and experimental purpose, while also considering photostability to ensure optimal results.
Preparation Steps for BODIPY Staining Solutions
- Solvent selection: Since BODIPY dyes have limited solubility in aqueous solutions, they are usually pre-dissolved in DMSO, ethanol, or small amounts of organic solvents to ensure complete dissolution without particle formation. For hydrophilic or lipophilic BODIPY derivatives, solvent selection should be optimized based on experimental requirements before further dilution into PBS or culture media.
- Dilution to working concentration: Pre-dissolved BODIPY dyes must be diluted to appropriate working concentrations depending on experiment type and sample characteristics. Overly high concentrations may cause quenching or nonspecific staining, while too low concentrations may result in weak signals. Proper adjustment ensures clear imaging and accurate quantification, suitable for bodipy staining lipid droplets, adipocytes, or cell lines.
- Light-protected storage: Although BODIPY dyes are relatively photostable, long-term light exposure may still cause signal decay. Staining solutions should be stored in light-protected containers at low temperature (e.g., 4°C), avoiding repeated freeze-thaw cycles to maintain stability and uniformity.
- Mixing before use: Solutions should be well mixed before application to ensure uniform dye distribution. For tissue sections or lipid droplet staining experiments, a homogeneous solution helps achieve consistent signals, avoiding local over- or under-staining, and ensures accuracy in bodipy staining imaging and flow cytometry analysis.
Recommended Concentrations for Different Sample Types
- Cell cultures or cell lines: 0.1–2 μM, suitable for bodipy staining cell culture and cell line experiments, enabling live-cell dynamics and quantitative analysis.
- Fixed cells: 0.5–5 μM, following bodipy staining fixed cells protocol, ensuring stable labeling of membranes and organelles.
- Tissue sections: 1–10 μM, suitable for bodipy staining tissue (e.g., liver, brain, and other organ slices), combined with permeabilization and optimized incubation for uniform staining.
- Lipid droplets or adipocytes: 1–3 μM, suitable for bodipy staining lipid droplets and adipocytes, providing clear visualization of droplet number, size, and distribution.
What is the Standard Protocol for BODIPY Staining?
The BODIPY staining protocol needs to be optimized according to different experimental objects (live cells, fixed cells, tissue sections, etc.) to ensure the stability, reproducibility, and specificity of fluorescence signals. A standardized bodipy staining protocol helps researchers reduce background signals, improve the accuracy of quantitative analysis, and facilitate comparison of results across different laboratories.
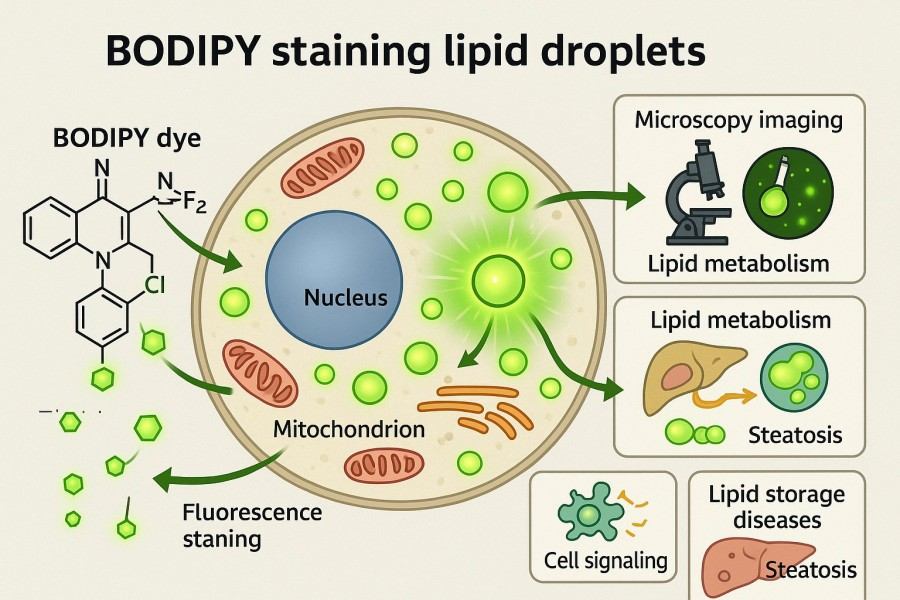 Fig. 2. BODIPY staining lipid droplets (BOC Sciences Authorized).
Fig. 2. BODIPY staining lipid droplets (BOC Sciences Authorized).
BODIPY Staining in Cell Culture and Cell Lines
BODIPY staining is widely used in cell culture and cell line experiments to monitor lipid droplets, membrane structures, and oxidative stress conditions. The basic workflow is as follows:
- Cell preparation: Culture target cells to appropriate density (typically 70–80% confluency). Remove the culture medium to avoid over-confluence, which may affect dye penetration.
- Addition of staining solution: Add pre-prepared bodipy staining solution at a recommended concentration of 0.1–2 μM, incubating at 37°C for 15–30 minutes. For oxidative stress experiments, C11-BODIPY can be used for real-time detection.
- Washing step: Gently wash 2–3 times with PBS to remove unbound or free dye and reduce background interference.
- Imaging and analysis: Observe cellular structures using fluorescence microscopy or perform quantitative analysis with flow cytometry. This method is commonly applied in bodipy staining cell line and bodipy staining cell culture research.
Notes: Avoid excessive incubation times to prevent cell death or nonspecific binding.
BODIPY Staining for Fixed Cells
Fixed cell staining is often used in structural studies and co-localization experiments, especially for histology and immunofluorescence. The standard bodipy staining fixed cells protocol is as follows:
- Fixation: Fix cells with 4% paraformaldehyde at room temperature for 15 minutes to preserve structural integrity.
- Washing step: Wash 2–3 times with PBS to completely remove residual fixatives and prevent interference with fluorescence signals.
- Staining step: Add BODIPY working solution at 0.5–5 μM, incubate in the dark for 20–60 minutes. Adjust concentration and time according to experimental needs; for lipid droplet studies, lower concentrations are often more suitable.
- Mounting and imaging: After PBS washing, apply an anti-fade mounting medium and image using confocal or fluorescence microscopy.
Notes: Pay attention to permeabilization conditions; different buffers or permeabilizing agents affect dye penetration efficiency.
BODIPY Staining in Tissue Sections
In tissue section experiments, BODIPY staining can be applied to study lipid metabolism, pathological states, and organelle distribution in the liver, brain, and other organs. The common bodipy staining tissue protocol is as follows:
- Section pretreatment: Paraffin sections require deparaffinization and rehydration, while frozen sections can be directly washed with PBS. Permeabilizing agents may be added if necessary to improve dye penetration.
- Staining step: Incubate sections in 1–10 μM BODIPY staining solution, protected from light, for 30–60 minutes.
- Washing and mounting: Wash sections 2–3 times with PBS, apply an anti-fade mounting reagent to maintain long-term signal stability.
- Imaging application: Combined with multichannel fluorescence labeling, BODIPY staining enables simultaneous observation of lipid droplets, blood vessels, and organelles, supporting studies such as bodipy staining in brain tissue and bodipy staining liver.
Notes: Tissue thickness and permeabilization significantly affect staining uniformity and should be optimized for different samples.
How to Perform BODIPY Staining on Specific Samples?
BODIPY staining has high adaptability across different types of biological samples, but its protocol needs to be optimized depending on the research object (lipid droplets, immune cells, bacteria, brain tissue, liver, etc.). Below are detailed applications and considerations for common experimental samples.
Staining Lipid Droplets and Adipocytes
Lipid droplets and adipocytes are classic targets for BODIPY staining, particularly with lipophilic derivatives.
- Dye selection: BODIPY 493/503 specifically binds to neutral lipids, commonly used in bodipy staining lipid droplets and bodipy staining adipocytes protocols. C11-BODIPY is suitable for studying lipid peroxidation and intracellular oxidative stress.
- Workflow:
- Wash cells with PBS to remove culture medium and serum lipids.
- Add 1–3 μM BODIPY working solution and incubate at 37°C, protected from light, for 15–30 minutes.
- Wash 2–3 times with PBS to reduce nonspecific binding.
- Applications: Used in research on lipid metabolism disorders, obesity, diabetes, and in drug screening experiments to analyze drug effects on lipid droplet formation and breakdown.
Notes: Over-incubation may lead to nonspecific cytoplasmic staining. Pre-experiments are recommended to determine the best concentration and incubation time.
BODIPY Staining of Macrophages and Bacteria
BODIPY is applicable not only to mammalian cells but also to immune cells and microorganisms.
- Macrophages:
- BODIPY derivatives can label lipid droplets or exogenous particles engulfed by macrophages.
- Frequently used to study foam cell formation in atherosclerosis, revealing the interplay between inflammation and lipid metabolism.
- Recommended staining concentration: 1–2 μM, incubation for 20–40 minutes.
- Bacteria:
- By selecting BODIPY probes with specific functional groups, bodipy staining of bacteria can be performed to trace bacterial membranes or intracellular lipid metabolism.
- In infection models, BODIPY staining can be combined with fluorescence imaging to observe macrophage phagocytosis of bacteria.
Notes: The bacterial cell wall has poor permeability, requiring optimized permeabilization steps or the use of hydrophilic BODIPY derivatives.
Applications in Brain Tissue and Liver Sections
In complex tissues, BODIPY staining reveals metabolic and pathological changes, making it highly valuable for disease research.
- Brain tissue:
- In bodipy staining in brain tissue, BODIPY is used to label neurons, lipid droplets, and lipid distribution in synaptic regions.
- Commonly applied in studies of neurodegenerative diseases such as Alzheimer's and Parkinson's, helping uncover links between lipid metabolism abnormalities and neuronal damage.
- Recommended use: low concentrations (1–5 μM) with extended incubation times (30–60 minutes) to ensure dye penetration.
- Liver sections:
- In bodipy staining liver studies, BODIPY is used to detect steatosis, lipid droplet accumulation, and drug-induced liver injury.
- Paraffin sections require deparaffinization and rehydration, while frozen sections can be directly stained.
- Typical staining concentration: 2–10 μM, incubation for 40–60 minutes.
Notes: Tissue thickness and permeabilizing agent selection are critical for staining results and must be optimized according to sample characteristics.
How to Quantify BODIPY Staining Results?
BODIPY staining not only provides intuitive fluorescence signals but also enables quantitative research through various imaging and analytical methods. Quantitative analysis helps researchers compare differences between different treatment groups, thereby providing reliable data support for studies in lipid metabolism, drug screening, cell damage, and disease models.
Imaging Techniques for Quantitative Analysis
In terms of imaging techniques, confocal microscopy is the most common tool, as it provides high-resolution images and allows precise localization of BODIPY fluorescence signals through optical sectioning, making it suitable for analyzing the spatial distribution of lipid droplets and their relationship with organelles. Widefield fluorescence microscopy, although slightly lower in resolution, offers advantages in rapid screening of large samples and high-throughput drug experiments, particularly when quantifying large populations of cells. For single-cell quantitative analysis, flow cytometry is the ideal choice. Bodipy staining flow cytometry can simultaneously measure the fluorescence intensity of thousands to millions of cells, enabling population-level comparisons of lipid droplet content or lipid peroxidation. Regardless of the imaging method used, consistent excitation wavelengths, exposure times, and imaging parameters are essential to ensure comparability of quantitative results.
Software Tools for BODIPY Staining Quantification
In the data analysis stage, the choice of image processing software is critical. ImageJ (FIJI), due to its open-source nature and flexible plugin system, is the most widely used tool. Researchers can use it to measure fluorescence intensity, lipid droplet area, and count, as well as perform large-scale data analysis through batch processing functions. CellProfiler is better suited for large datasets requiring automated processing, as it can identify different cellular features based on preset rules or machine learning algorithms, making it especially useful in bodipy staining imaging with complex multicellular images. For tissue sections and three-dimensional imaging experiments, commercial software such as Imaris or MetaMorph provides 3D reconstruction functions, helping researchers analyze lipid droplet distribution patterns in brain or liver tissue. The proper application of these software tools can effectively enhance the quantitative level of BODIPY staining results.
Troubleshooting Quantification Challenges
During the quantification of BODIPY staining results, researchers often encounter the following challenges and their corresponding solutions:
- Excessive background signals: May result from excessively high dye concentration or insufficient washing. Reduce BODIPY concentration appropriately and increase PBS washing steps to minimize nonspecific binding.
- Uneven signals: Commonly caused by incomplete dye dissolution or uneven sample processing. Fully dissolve the dye in DMSO or ethanol, and gently mix during incubation to significantly improve this issue.
- Poor reproducibility: Inconsistent conditions across different experimental batches often lead to discrepancies. Strictly standardize the bodipy staining protocol, including incubation time, dye concentration, and microscope exposure parameters.
- Bias in quantitative analysis: Manual selection of ROI (regions of interest) may introduce subjective errors. Use automated software such as ImageJ or CellProfiler for batch analysis to improve objectivity and consistency of results.
What Challenges Are Common in BODIPY Staining and How to Overcome Them?
In BODIPY staining experiments, researchers often encounter issues affecting signal quality and data reproducibility. Without optimization, these issues may result in blurred fluorescence images, excessive background signals, or significant variability between experiments. Understanding and addressing these challenges is essential to improving the reliability of the bodipy staining protocol.
Reducing Background and Improving Signal Clarity
Excessive background signals are among the most common issues in BODIPY staining, significantly reducing the signal-to-noise ratio of imaging and complicating quantitative analysis. Causes of background enhancement usually include overly high dye concentrations, residual unbound dye, or poor mounting conditions. To resolve this issue, researchers can first optimize bodipy staining concentration to avoid fluorescence quenching or over-staining. Second, increasing PBS or buffer washing steps after staining helps remove unbound free dye, thereby reducing nonspecific signals. Additionally, selecting appropriate mounting media and anti-fade reagents not only improves signal clarity but also extends imaging time, preventing signal loss during prolonged exposure. These measures allow researchers to obtain higher-quality and more stable BODIPY imaging results.
Ensuring Consistency Across Cell Lines and Tissue Types
Another common challenge is inconsistency in BODIPY staining performance across different cell lines and tissue types. For example, some cell lines may exhibit weak fluorescence due to poor membrane permeability and low dye penetration efficiency, while lipid droplet-rich cells or tissue sections may show oversaturated signals. To address these differences, researchers need to flexibly adjust staining conditions according to sample characteristics. For cell culture and cell lines, gradually optimizing dye concentration and incubation time can achieve the best staining effect. In tissue section experiments, additional pretreatments such as deparaffinization, rehydration, and permeabilization must be considered to ensure uniform dye penetration. By establishing a standardized bodipy staining protocol and maintaining consistency across experiments, batch-to-batch variability can be minimized, yielding more reliable comparative results.
Support Services for BODIPY Staining from BOC Sciences
BOC Sciences is dedicated to providing high-quality BODIPY fluorescence products and comprehensive technical support for researchers worldwide. Our services cover customized synthesis of BODIPY dyes, screening of diverse derivatives, and optimization of experimental protocols and application guidance, addressing a wide range of needs including cell culture, fixed cells, and tissue sections. To tackle challenges in understanding the bodipy staining principle, establishing a reliable bodipy staining protocol, and performing accurate signal quantification, our expert team delivers tailored solutions that enhance experimental reproducibility and data reliability.
Diverse Fluorescent Product Supply
- High-purity, structurally stable BODIPY dyes and derivatives covering excitation and emission wavelengths from blue to red.
- A wide variety of products to meet needs in cell staining, membrane labeling, lipid imaging, and more.
- All products undergo strict quality control to ensure batch-to-batch consistency and provide reliable fluorescence signals.
- Researchers can select BODIPY derivatives with different functional modifications to achieve specific imaging effects.
Customized Dye Modification Services
- Introduction of specific functional groups (e.g., carboxyl, amino, or active esters) into BODIPY dye molecules for conjugation with target molecules.
- Optimization of optical properties based on experimental needs, such as tuning excitation/emission wavelengths, improving quantum yield, or enhancing photostability.
- Personalized structural modifications and derivative design to ensure optimal dye performance in specific environments or cell types.
- Small-scale sample validation available to reduce R&D risks and accelerate experimental progress.
Conjugation and Labeling Services
- Efficient conjugation of BODIPY dyes with biomolecules such as proteins, antibodies, nucleic acids, and lipids to achieve specific labeling.
- Multiple conjugation strategies available, including covalent binding and specific affinity, to meet diverse experimental needs.
- Conjugated products are strictly purified and validated to ensure labeling efficiency and fluorescence stability.
- Support for customized multicolor labeling solutions, enabling multiplex imaging and analysis of complex biological systems.
Multifunctional Probe Development
- Development of BODIPY probes with environment-responsive properties, such as pH-sensitive, ion-selective, or redox-responsive probes.
- Probe designs targeting specific subcellular structures or microenvironments for precise imaging and functional monitoring.
- Support for structural optimization and tuning of optical performance to improve signal sensitivity and stability.
- Comprehensive development support from design to validation, helping research teams quickly obtain customized functional probes.
Do You Need A Consultation?
BOC Sciences integrates cutting-edge fluorescence technologies to accelerate your research, driving next-generation solutions for drug discovery and diagnostics.
Transform Your Studies with Cutting-Edge Fluorescent Products
| Catalog | Name | CAS | Inquiry |
|---|---|---|---|
| F01-0064 | meso-CH2Br-BODIPY | 216434-81-0 | Bulk Inquiry |
| F01-0053 | 8(4'-bromophenyl)-1,3,5,7-tetramethyl-BODIPY | 850534-66-6 | Bulk Inquiry |
| F01-0221 | BODIPY Green 8-P2M | 929679-22-1 | Bulk Inquiry |
| F01-0166 | BODIPY 493/503 NHS Ester | 216961-98-7 | Bulk Inquiry |
| F01-0074 | 2,6-Diiodo-1,3,5,7,8-pentaethyl-BODIPY | 1031443-55-6 | Bulk Inquiry |
| F01-0012 | 3-Bodipy-propanoic acid | 165599-63-3 | Bulk Inquiry |
| F01-0065 | 8-(4-Anilino) Bodipy | 321895-93-6 | Bulk Inquiry |
| F01-0220 | BODIPY TR-X NHS Ester | 217190-13-1 | Bulk Inquiry |
| F01-0152 | BODIPY-X-Alkyne | 1173281-82-7 | Bulk Inquiry |
| F01-0154 | BODIPY FL acid | 126250-45-1 | Bulk Inquiry |
High-Performance Fluorescent Tools for Your Research
- Alexa Fluor Bright, stable dyes for sensitive biosensing applications.
- Cyanine Versatile dyes used in biosensing and nucleic acid detection.
- Rhodamine Strong fluorescence, commonly used in protein and DNA sensing.
- Fluorescent Protein Used for live-cell imaging and real-time biosensing.
More About BODIPY Dyes
Online Inquiry

