BODIPY Design, Synthesis and Functionalization: High-Performance BODIPY Derivatives
In modern biochemistry and molecular imaging research, BODIPY (boron-dipyrromethene) dyes have become researchers' preferred fluorescent tools due to their excellent photophysical properties, chemical stability, and versatile derivatization potential. However, the design, synthesis, and functionalization of BODIPY dyes still face many challenges, including yield optimization, solubility control, and specific biomarking. This article provides a comprehensive analysis of BODIPY dyes—from the fundamentals, synthesis mechanisms, derivative development, and modification strategies to practical applications—highlighting their critical role in research and offering practical solutions for scientists.
What is BODIPY and Why is it Important in Fluorescent Dye Chemistry?
BODIPY dyes are a class of fluorescent compounds with stable structures and outstanding optical properties, widely applied in molecular imaging and biomarking. Their unique photophysical features and chemical modifiability make them essential tools for cell imaging, membrane studies, and fluorescent probe development. Therefore, understanding the basic properties of BODIPY is the foundation for subsequent synthesis and functionalization studies.
Overview of BODIPY Dyes and Their Unique Photophysical Properties
BODIPY dyes are fluorescent dyes based on pyrrole–boron complexes, characterized by very high quantum yields, narrow emission spectra, and excellent photostability. These properties give them irreplaceable advantages in cell imaging, molecular probe development, and fluorescent sensor research. Compared with traditional fluorescent dyes, BODIPY exhibits low background fluorescence and high chemical stability, enabling high signal-to-noise ratio imaging in complex biological systems. Its core scaffold is easily modifiable, allowing wavelength tuning and functional derivative development through side-chain modifications, thereby meeting diverse research needs.
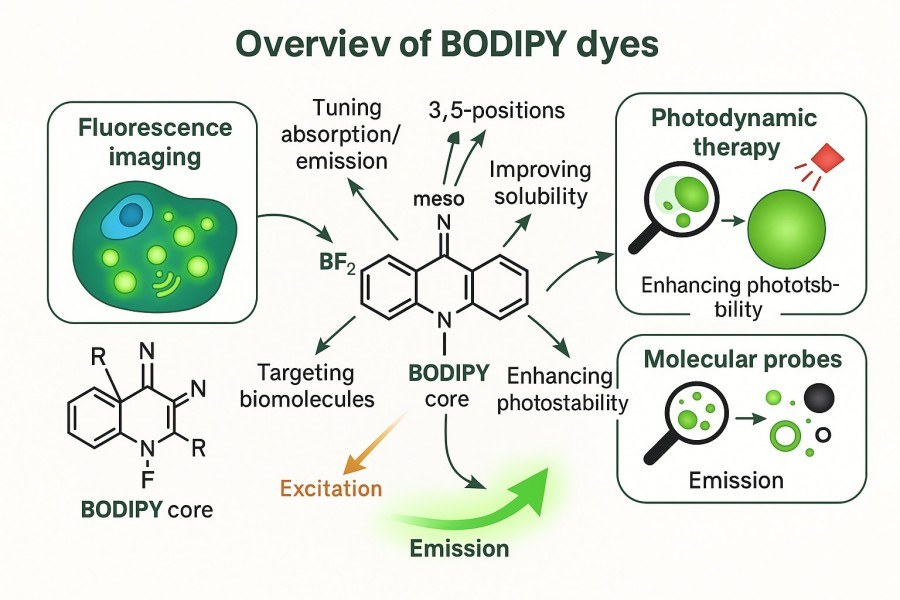 Fig. 1. Overview of BODIPY dyes (BOC Sciences Authorized).
Fig. 1. Overview of BODIPY dyes (BOC Sciences Authorized).
Key Challenges in BODIPY Design and Functionalization
Although BODIPY dyes are widely favored in fluorescence research for their high quantum yields, narrow emission spectra, and excellent photostability, their synthesis and application still face significant challenges that directly impact dye performance and experimental reproducibility. Understanding and addressing these issues is essential for designing high-performance BODIPY derivatives and optimizing scientific experiments.
- Yield and Purity Control: During multi-step synthesis, condensation, oxidation, and boron coordination reactions can generate by-products or unreacted intermediates, reducing overall yield. Insufficient product purity affects fluorescence performance and subsequent functionalization efficiency. Thus, optimizing reaction conditions, selecting appropriate solvents and catalysts, and applying purification methods such as column chromatography or HPLC are necessary to ensure high yield and purity.
- Solubility and Stability Issues: Lipophilic or highly conjugated BODIPY derivatives tend to aggregate or precipitate in aqueous systems, causing fluorescence quenching. Moreover, BODIPY may undergo photodegradation or thermal decomposition under strong light or high temperature. Introducing hydrophilic substituents, PEG chains, or lipid derivatives can improve solubility, dispersibility, and photothermal stability to meet bioimaging requirements.
- Functionalization Difficulties: Achieving specific biomarking or membrane binding often requires multi-step chemical modifications, such as amidation, click chemistry, or thiol coupling. Functionalization must preserve fluorescence performance and labeling selectivity, avoiding spectral shifts or nonspecific binding. Careful design of modification strategies and reaction optimization are crucial for obtaining high-performance derivatives.
- Wavelength Tuning Limitations: The absorption and emission wavelengths of BODIPY are highly dependent on its core scaffold and substituent structures. Introducing electron-donating/withdrawing groups, cyclic substituents, or N/O substitutions can tune spectra, but excessive conjugation may lead to aggregation and quenching. Rational substituent design enables wavelength control while maintaining strong fluorescence and stability.
BODIPY Synthesis Mechanism and Key Procedures
The unique optical properties and chemical stability of BODIPY dyes stem from their precisely designed molecular framework, making mastery of their synthesis mechanisms crucial for obtaining high-performance dyes. BODIPY synthesis typically involves pyrrole precursor preparation, boron coordination, and side-chain functionalization, with each step critically influencing fluorescence properties and purity. A detailed understanding of each reaction mechanism effectively guides experimental optimization, improving yield and stability.
General BODIPY Synthesis Procedure: Steps and Reaction Conditions
The entire synthesis process requires strict control of reaction temperature, solvents, and moisture to prevent side reactions and reduced yields. The typical procedure includes:
- Pyrrole Precursor Preparation: The first step is acid-catalyzed condensation of aldehydes or ketones with pyrrole to generate intermediates. This step is sensitive to reaction temperature, acid catalyst type, and solvent conditions. Optimizing these parameters enhances intermediate yields and reduces by-products, laying the foundation for subsequent boron coordination.
- Boron Coordination Reaction: The pyrrole intermediate reacts with boron reagents (e.g., BF₃·OEt₂), forming a stable BODIPY core. Strictly dry conditions and controlled reaction times are required to avoid degradation. Proper solvent and temperature selection significantly enhances fluorescence properties and yield.
- Side-Chain Modification: Introducing carboxyl, amino, alkyl, or aryl substituents to the BODIPY core imparts functional properties such as water solubility tuning, membrane affinity, or biomarking capability. These modifications are usually carried out under dry, inert atmospheres to prevent structural damage or fluorescence loss.
- Purification and Characterization: Products are typically purified by column chromatography or HPLC to meet research-grade purity standards. NMR, MS, and UV-Vis spectroscopy confirm structural integrity and optical characteristics, providing a reliable foundation for further functionalization and applications.
Detailed BODIPY Synthesis Mechanism
The synthesis mechanism centers on the sequence and condition control of pyrrole–aldehyde condensation and boron coordination. For example, in aza-BODIPY synthesis, introducing nitrogen atoms at pyrrole positions significantly red-shifts emission wavelengths, but requires mild conditions to prevent scaffold cleavage or spectral loss. The general mechanism includes:
- Condensation Reaction: Two pyrrole molecules and one aldehyde undergo acid-catalyzed condensation to form a dihydropyrrole intermediate. Reaction selectivity and yield are highly dependent on acid concentration, solvent polarity, and temperature. Precise control reduces by-product formation.
- Oxidation/Dehydrogenation: Using oxidants such as DDQ or MnO₂, the intermediate is dehydrogenated into a dipyrromethene. This step is critical for establishing conjugation and directly affects optical performance, requiring mild and uniform conditions to prevent structural degradation.
- Boron Coordination: Under dry, inert conditions, the dipyrromethene reacts with BF₃·OEt₂ to form a stable BODIPY core. Boron coordination stabilizes the dye structure and imparts strong fluorescence. Controlling moisture and temperature is key to achieving high yields.
Strategies for Improving Yields and Purity During BODIPY Dye Synthesis
- Optimized Solvent Systems: Anhydrous solvents such as chloroform, THF, or DMSO improve solubility, reduce side reactions, and enhance selectivity, ensuring high yields.
- Precise Temperature and Time Control: Excessive temperature or prolonged reaction increases by-products and reduces performance. Tight control ensures higher yield and structural integrity.
- Dry and Inert Atmosphere Protection: Boron coordination and side-chain modifications must be conducted under anhydrous, inert conditions to prevent hydrolysis or side reactions, ensuring stability and purity.
- Efficient Purification Methods: Combining column chromatography with HPLC significantly improves purity by removing by-products and unreacted intermediates, delivering research-grade BODIPY dyes for reliable functionalization and applications.
BODIPY Staining Services by BOC Sciences
| Solutions | Capabilities |
|---|---|
| Cell Imaging | Enabled with high-purity, photostable BODIPY dyes delivering precise subcellular localization and dynamic process visualization. |
| Lipid Staining | Achieved through tailored BODIPY conjugates optimized for membrane insertion, lipid tracking, and metabolic studies. |
| Cell Staining | Supported by versatile BODIPY derivatives ensuring strong fluorescence signals and compatibility with live or fixed cells. |
| Enzyme Staining | Realized with functionalized BODIPY probes designed for activity-based labeling and pathway analysis. |
| Protein Staining | Facilitated via advanced bioconjugation strategies providing stable BODIPY–protein labeling with preserved bioactivity. |
BODIPY Design Considerations for High-Performance Derivatives
The performance of BODIPY dyes depends on precise molecular design, including scaffold structure, substituent selection, and functionalization strategies. Rational design optimizes optical properties, solubility, stability, and biocompatibility, meeting diverse scientific needs. When developing new BODIPY derivatives, considering absorption/emission tuning, membrane affinity, conjugation sites, and environmental sensitivity is crucial for experimental success.
Core Structure Optimization
Adjusting substituent positions, conjugation length, or cyclization in the BODIPY core enables precise control over absorption/emission wavelengths, quantum yields, and photostability. Rational scaffold design enhances fluorescence intensity, reduces photobleaching, and provides viable chemical sites for further functionalization, facilitating multicolor imaging and photosensitive probe development.
Substituent and Functional Group Selection
Introducing hydrophilic, hydrophobic, or bioactive substituents improves solubility, membrane binding, and targeting capabilities. Functional groups such as amino, carboxyl, azide, or thiol ensure efficient conjugation and labeling while preserving optical performance, offering reliable tools for live-cell imaging, membrane research, and probe development.
Photophysical and Chemical Performance Tuning
Designing derivatives requires balancing optical and chemical performance, including quantum yield, spectral narrowness, photobleaching resistance, and environmental responsiveness. By tuning substituent electronic properties, scaffold conjugation, and conjugation strategies, researchers can develop dyes tailored for specific biological systems or multicolor imaging experiments, delivering reliable fluorescent tools.
BODIPY Derivative Synthesis: Expanding Functionality
The diverse derivatives of BODIPY, achieved through core scaffold modification or molecular conjugation, significantly expand functionality. These derivatives not only improve solubility and membrane affinity but also provide specific biomarking capabilities. Different functionalization strategies allow precise tuning of optical properties and biocompatibility according to experimental needs, supporting applications in cell imaging, membrane studies, and molecular probe development.
| BODIPY Derivative | Functional Features | Main Applications | Advantages |
|---|---|---|---|
| BODIPY cholesterol | Inserts into lipid bilayers | Membrane fluidity and lipid raft studies | High membrane affinity, stable fluorescence |
| BODIPY amine | Amino group for conjugation | Protein labeling and bioconjugation | Supports acylation or reductive coupling, high specificity |
| BODIPY azide | Azide group | Click chemistry conjugation | Fast reaction, versatile functional expansion |
| BODIPY carboxylic acid | Carboxyl group for conjugation | Antibody and peptide labeling | Forms amide bonds easily, high labeling efficiency |
| BODIPY ceramide | Lipid backbone | Membrane dynamics and lipid metabolism | Controllable membrane insertion, supports cellular localization |
| BODIPY fatty acid | Modified fatty acid chain | Membrane studies and metabolic tracking | Adjustable polarity and chain length, optimized insertion efficiency |
| BODIPY cyclopamine | Hedgehog pathway targeting | Signal pathway studies | Strong targeting, suitable for specific pathway imaging |
| BODIPY maleimide | Thiol-specific binding | Hapten and protein labeling | Highly selective protein binding, controlled fluorescence |
| BODIPY NHS ester | Active ester group | Antibody and peptide labeling | High specificity amino coupling, convenient for imaging |
BODIPY Modification Strategies for Advanced Research
In advanced scientific applications, BODIPY dyes are often required to meet higher standards in terms of optical performance, targeting specificity, and chemical stability. Through fine chemical modifications, bioconjugation techniques, and solubility optimization strategies, the functionality of BODIPY can be expanded to achieve multicolor imaging, molecular probe development, and studies of complex biological systems. These strategies provide researchers with flexible design approaches, ensuring that dye performance aligns closely with experimental needs.
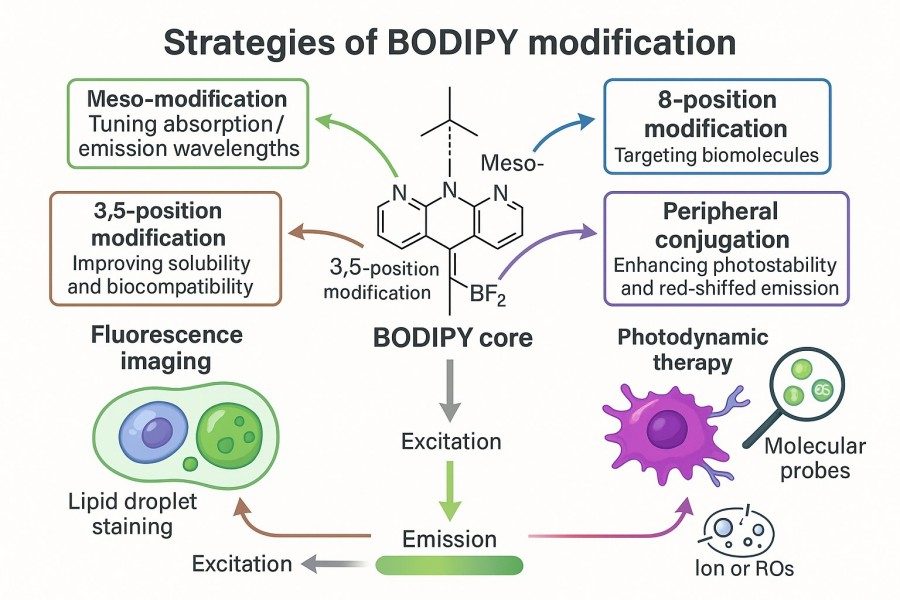 Fig. 2. Strategies of BODIPY modification (BOC Sciences Authorized).
Fig. 2. Strategies of BODIPY modification (BOC Sciences Authorized).
Chemical Modifications to Tune Absorption and Emission Wavelengths
By introducing conjugated substituents, nitrogen or oxygen atoms, and cyclic structures into the BODIPY core, absorption and emission wavelengths can be precisely tuned, enabling multicolor fluorescence modulation from the visible to the near-infrared region. This chemical modification strategy not only supports multiplex fluorescence imaging but also facilitates the design of photosensitive probes, energy transfer systems, and photodynamic agents. Through rational design of substituent positions and electronic properties, researchers can optimize spectral characteristics, quantum yield, and photostability, providing greater flexibility for advanced imaging and molecular probe applications.
Bioconjugation Techniques for Target-Specific Imaging Applications
BODIPY dyes can be conjugated to biomolecules through multiple bioconjugation strategies, including amide bond formation, thiol-click chemistry, and carbohydrate modifications. These methods enable stable linkage of BODIPY to proteins, nucleic acids, or lipids, allowing highly selective imaging and molecular tracking. By controlling conjugation sites and efficiencies, the optical properties of the dye can be preserved while maximizing biological activity. These strategies provide reliable tools for dynamic cell imaging, subcellular localization studies, and molecular interaction analysis, significantly broadening the scope of BODIPY applications in life sciences research.
Overcoming Solubility and Stability Issues in BODIPY Derivative Synthesis
BODIPY derivatives often suffer from poor solubility in aqueous systems due to their high conjugation and lipophilic character, and they may degrade under light or heat exposure. To address these issues, hydrophilic substituents, PEG chains, or phospholipid derivatives can be introduced to improve solubility, dispersibility, and photo/thermal stability. This ensures homogeneous distribution of the dye in complex biological systems and stable fluorescence signals in cell imaging, membrane studies, and in vitro experiments, providing reliable support for advanced research applications.
Applications of BODIPY Staining in Biological Research
With their high quantum yield, excellent photostability, and tunable spectral properties, BODIPY dyes have been widely applied in biological research. Their diverse functional derivatives enable researchers to perform organelle localization, membrane structure analysis, molecular interaction tracking, and metabolic monitoring. Through rational design of BODIPY derivatives and conjugation strategies, highly specific and sensitive fluorescence imaging can be achieved in live cells or in vitro systems, supporting visualization of complex biological processes and signaling pathways. The flexibility and reliability of BODIPY make it an indispensable tool in life sciences.
Cellular Imaging and Localization
Due to their brightness and photostability, BODIPY dyes enable precise subcellular localization in both live and fixed cells. They can label organelles such as mitochondria, endoplasmic reticulum, and nuclei, allowing researchers to monitor dynamic processes in real time, including material transport, energy metabolism, and signal transduction. By selecting appropriate functional derivatives, BODIPY dyes can bind to specific proteins or membrane structures, offering reliable tools for organelle visualization, subcellular localization, and disease-related mechanism studies.
Lipid and Membrane Studies
Lipid-conjugated BODIPY derivatives have unique advantages in membrane studies, supporting research on membrane fluidity, lipid raft distribution, and lipid metabolism. Their stable fluorescence enables real-time tracking of lipid localization and rearrangement during membrane trafficking. By tuning fatty acid chain length or polarity, insertion efficiency and selectivity into membranes can be optimized, providing powerful tools for studying lipid metabolic dynamics, membrane protein interactions, and membrane structure visualization.
Tracking Molecular Interactions
With their high quantum yield, narrow emission spectra, and multicolor compatibility, BODIPY dyes are ideal for monitoring protein–protein, protein–nucleic acid, and multiprotein complex interactions dynamically. Their stable fluorescence enables accurate tracking of molecular interactions in live cells or in vitro systems, supporting pathway analysis, drug screening, and mechanism studies. By designing proper conjugation strategies, BODIPY can be used to develop targeted probes and multiplex labeling systems, offering reliable tools for complex biomolecular research.
Fluorescent Probes for Metabolic Studies
BODIPY derivatives can be engineered as metabolism-specific fluorescent probes for tracking dynamic changes in lipids, carbohydrates, or small-molecule metabolites. Through functional modifications, dyes can be localized in cells or tissues in real time, helping researchers monitor energy metabolism, fatty acid oxidation, or intracellular metabolite flux. These probes provide powerful visualization tools for studying metabolic regulation and disease mechanisms.
How BOC Sciences Can Support Your BODIPY Dye Projects?
In the field of BODIPY fluorescent dye design and application, BOC Sciences provides comprehensive support for researchers and enterprises through strong synthesis and functionalization capabilities. Our extensive library of high-quality BODIPY products covers basic dyes, functionalized derivatives, and probes with specific spectral ranges, meeting diverse needs in cell imaging, membrane studies, and molecular interaction research. Meanwhile, our custom synthesis services support targeted substituent design, conjugated derivative development, and construction of complex structures, ensuring that researchers obtain exclusive dyes tailored to their experimental objectives.
Diverse High-Quality BODIPY Products
- A comprehensive library of BODIPY dyes, including base dyes, functional derivatives, and products with defined absorption/emission wavelengths, meeting diverse research demands.
- All products undergo strict quality control to ensure high purity and stable fluorescence performance, suitable for cell imaging, membrane studies, and molecular probe development.
- Reliable results across different experimental systems, whether in aqueous biological environments or organic solvents.
Custom Dye Synthesis and Functionalization Services
- Custom synthesis services tailored to client needs, including specific substituents, conjugated derivatives, or unique core structures.
- Support for multistep chemical modifications to develop functional derivatives, such as hydrophilic modifications, membrane-targeted optimization, or environment-sensitive dyes.
- Flexible customization strategies that meet special requirements for spectral properties, solubility, and stability, improving experimental control and efficiency.
Bioconjugation and Labeling Solutions
- BODIPY conjugation and labeling services for proteins, antibodies, nucleic acids, and lipids, ensuring efficient and stable linkage with minimal side reactions.
- Techniques including amide coupling, thiol-click chemistry, and NHS ester conjugation preserve dye optical properties while maintaining biological activity.
- Support for targeted imaging, molecular interaction studies, and live-cell tracking, providing reliable tools for life sciences research.
Development of Multi-Functional Probes for Advanced Applications
- Development of multifunctional BODIPY probes, such as lipid-fluorescent dual probes, environment-sensitive dyes, or multicolor imaging derivatives.
- Applications include membrane structure analysis, metabolic tracking, signaling pathway studies, and multiplex labeling experiments for visualizing complex biological processes.
- Integration of custom synthesis and bioconjugation technologies provides one-stop solutions for advanced imaging studies and innovative research.
Do You Need A Consultation?
BOC Sciences integrates cutting-edge fluorescence technologies to accelerate your research, driving next-generation solutions for drug discovery and diagnostics.
Transform Your Studies with Cutting-Edge Fluorescent Products
| Catalog | Name | CAS | Inquiry |
|---|---|---|---|
| F01-0155 | BODIPY Fl C5-Ceramide | 133867-53-5 | Bulk Inquiry |
| F01-0158 | BODIPY TR methyl ester | 150152-63-9 | Bulk Inquiry |
| F01-0161 | BODIPY 558/568 C12 | 158757-84-7 | Bulk Inquiry |
| F01-0162 | BODIPY FL Prazosin | 175799-93-6 | Bulk Inquiry |
| F01-0163 | BODIPY FL Thapsigargin | 216571-99-2 | Bulk Inquiry |
| F01-0045 | BODIPY 505/515 | 21658-70-8 | Bulk Inquiry |
| F01-0178 | BODIPY TR Cadaverine | 217190-24-4 | Bulk Inquiry |
| F01-0046 | Bodipy C12-Ceramide | 1246355-58-7 | Bulk Inquiry |
| F01-0041 | Olaparib-bodipy FL | 1380359-84-1 | Bulk Inquiry |
| F01-0121 | 3-styryl-BODIPYs | 1321616-68-5 | Bulk Inquiry |
High-Performance Fluorescent Tools for Your Research
- Alexa Fluor Bright, stable dyes for sensitive biosensing applications.
- Cyanine Versatile dyes used in biosensing and nucleic acid detection.
- Rhodamine Strong fluorescence, commonly used in protein and DNA sensing.
- Fluorescent Protein Used for live-cell imaging and real-time biosensing.
More About BODIPY Dyes
Online Inquiry

