How to Modulate Photophysical, Photochemical, and Stability Properties of BODIPY Dyes?
BODIPY dyes have become essential tools in fields such as bioimaging, photodynamic therapy, sensor development, and materials science due to their excellent photophysical properties, high fluorescence quantum yields, and chemical modifiability. However, native BODIPY suffers from certain limitations in emission wavelength, Stokes shift, photostability, and photochemical activity, making it challenging to meet the diverse requirements of different applications. To achieve precise utilization of high-performance BODIPY dyes, researchers need to systematically optimize their photophysical properties, photochemical behavior, and chemical/photostability through molecular structural modification and environmental tuning.
What is BODIPY Dye and Why Modulate BODIPY Dye Properties?
BODIPY is a class of high-performance organic fluorescent dyes widely used in bioimaging, photodynamic therapy (PDT), sensors, and materials science due to their narrow emission bands, high molar absorptivity, and good chemical stability. Nevertheless, the native BODIPY scaffold still has limitations, and researchers often need to modulate its photophysical, photochemical, and stability properties through molecular structural modification to meet specific application requirements.
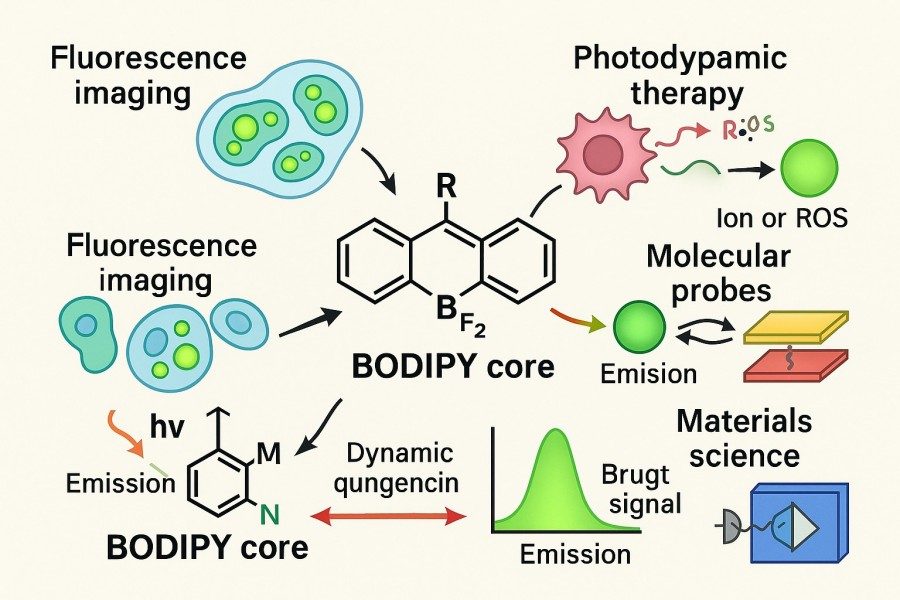 Fig. 1. BODIPY dyes uses (BOC Sciences Authorized).
Fig. 1. BODIPY dyes uses (BOC Sciences Authorized).
Core Advantages and Limitations of Native BODIPY
Native BODIPY offers high fluorescence quantum yields, narrow emission peaks, and strong photostability, making it an ideal foundation for many fluorescent probe designs. Its limitations, however, include small Stokes shifts, limited solubility, potential degradation in polar or biological environments, and restricted triplet quantum yields for photodynamic therapy. These drawbacks limit its performance in complex biological environments and advanced optical applications. Key limitations of native BODIPY include:
- Small Stokes shift: Can lead to self-absorption issues, affecting imaging resolution.
- Limited solubility: Often insufficient in aqueous and biological environments, requiring the introduction of hydrophilic groups.
- Restricted photochemical activity: Low triplet quantum yield limits its application in photodynamic therapy.
- Environmental sensitivity: Stability may be inadequate in polar solvents, oxidative conditions, or complex in vivo systems.
Application-Specific Performance Requirements
Precisely matching BODIPY performance to application needs is a major challenge for researchers. Different applications impose distinct requirements:
- Bioimaging: Requires high brightness, good water solubility, and low background signal for clear imaging.
- Photodynamic therapy: Needs efficient triplet formation and singlet oxygen generation for effective tumor ablation.
- Fluorescent sensing: Demands sensitive optical responses (fluorescence lifetime, intensity, or wavelength shifts) for detecting ions, pH, or small molecules.
- Materials and devices: Requires stable photoluminescent properties and controllable electronic energy levels for OLEDs, solar cells, or lasers.
Overview of Key Modifiable Parameters
Researchers commonly tune BODIPY dyes through the following aspects:
- Photophysical properties: Including absorption and emission wavelengths, fluorescence quantum yield, Stokes shift, and fluorescence lifetime. These parameters determine BODIPY's performance in imaging, probes, and materials.
- Photochemical behavior: Including intersystem crossing efficiency, triplet formation, and singlet oxygen generation, crucial for PDT and photocatalytic applications.
- Chemical and photostability: Dictates whether BODIPY can maintain stable signals in complex environments, affecting reliability in in vivo imaging and long-term experiments.
- Solubility and biocompatibility: Introducing hydrophilic groups or carriers can enhance performance in aqueous media and expand biomedical applications.
- Functionalization capacity: BODIPY offers abundant substitution sites for conjugation with targeting moieties, reactive groups, or other fluorescent modules, enabling more complex probe designs.
Tuning Photophysical Properties of BODIPY for Enhanced Fluorescence
Photophysical properties are central to the application performance of BODIPY dyes, encompassing absorption and emission spectra, fluorescence quantum yield, brightness, Stokes shift, and fluorescence lifetime. Depending on research contexts, researchers need to modulate these features through molecular design and chemical modification to achieve superior fluorescence performance. Particularly in bioimaging, fluorescent probe design, and advanced optical device applications, fine-tuning photophysical properties is often crucial for improving signal sensitivity, resolution, and reliability.
Strategies to Adjust Absorption and Emission Wavelengths of BODIPY
Tuning absorption and emission wavelengths is the first step in optimizing BODIPY performance. Native BODIPY typically absorbs and emits in the 500–600 nm range, while biomedical imaging favors the near-infrared (NIR, 650–900 nm) window to reduce tissue autofluorescence and improve penetration depth. Common approaches include:
- Extending π-conjugation: Introducing aromatic or vinyl substituents at the α or β positions of the BODIPY core can effectively red-shift absorption and emission spectra.
- Incorporating push-pull electronic groups: Adding electron donors (e.g., amino) and acceptors (e.g., cyano, nitro) at the meso position or aromatic rings can modulate emission energy levels via intramolecular charge transfer, achieving red or blue shifts.
- Molecular coupling strategies: Conjugating BODIPY with other fluorophores to form dimers or oligomers can produce broader absorption bands and controlled energy transfer.
These strategies enable researchers to tune BODIPY emission across the visible to NIR range, expanding its potential in in vivo imaging and deep-tissue detection.
Improving Quantum Yield and Brightness
Quantum yield and brightness determine the signal-to-noise ratio and detection sensitivity of BODIPY in imaging. While native BODIPY has relatively high quantum yield, fluorescence quenching may occur in polar environments or biological systems. Common optimization strategies include:
- Suppressing non-radiative decay: Introducing bulky substituents near the BODIPY core reduces molecular rotation and vibrational freedom, minimizing energy loss.
- Rigidifying the structure: Adding cyclic or bridged structures to the BODIPY scaffold increases rigidity, significantly enhancing fluorescence efficiency.
- Environmental control: Using polymer matrices, protein binding, or nanoparticle carriers improves the local environment, preventing solvent-induced fluorescence quenching.
These approaches allow BODIPY to maintain high brightness and provide reliable optical signals even under complex biological conditions or extreme experimental setups.
Tailoring Stokes Shift and Fluorescence Lifetime
Another notable limitation of BODIPY dyes is their small Stokes shift, usually only 10–20 nm. This can cause overlap between excitation and emission spectra, leading to self-absorption and reduced imaging quality. Strategies to overcome this include:
- Introducing strong push/pull electronic groups: Incorporating strong electron donors or acceptors at the meso position promotes intramolecular charge transfer (ICT), effectively increasing the Stokes shift.
- Constructing push-pull BODIPY: Introducing asymmetric substituents at different positions creates uneven electron distribution, achieving larger spectral shifts.
- Utilizing aggregation-induced emission (AIE) effects: Certain modifications induce larger Stokes shifts in the aggregated state, suitable for materials and nanoprobe development.
Additionally, fluorescence lifetime reflects the dynamics of the excited state and is particularly important for fluorescence lifetime imaging (FLIM). Extending conjugation or introducing rigid rings can prolong fluorescence lifetime, enabling time-resolved detection and enhancing probe utility in complex biological environments.
BODIPY Services by BOC Sciences
| Solutions | Capabilities |
|---|---|
| In Vivo Imaging | Enables high-contrast, long-lasting fluorescence for precise tracking of biological processes in living organisms. |
| Cell Imaging | Provides bright and stable signals for detailed visualization of cellular structures and dynamics. |
| FRET Microscopy | Supports efficient energy transfer measurements with customizable donor-acceptor BODIPY pairs. |
| Lipid Staining | Delivers selective, high-sensitivity labeling of lipid membranes for quantitative analysis. |
| Flow Cytometry | Offers robust, high-fluorescence dyes compatible with multi-parameter cytometric assays. |
Modifying Photochemical Behavior of BODIPY for Functional Applications
BODIPY dyes attract attention not only for their excellent photophysical properties but also for their photochemical behavior, such as excited-state dynamics, intersystem crossing efficiency, and singlet oxygen generation capacity, which directly determine their potential in PDT, photocatalysis, controllable probes, and energy conversion systems. Native BODIPY exhibits limited energy conversion efficiency between ground and excited states, particularly with low triplet formation and reactive oxygen generation, creating bottlenecks in the development of photosensitizers and photoresponsive probes. Therefore, molecular design and optimization of photochemical properties for specific applications are key to advancing BODIPY from fundamental research to functional applications.
Enhancing Intersystem Crossing for Triplet State Formation
Triplet state formation is central to many photochemical applications; for instance, PDT relies on triplet-excited products to generate reactive oxygen species, while photocatalytic systems depend on them for energy or electron transfer. Native BODIPY exhibits weak spin-orbit coupling, resulting in low intersystem crossing (ISC) efficiency and limited triplet quantum yield. Common strategies to enhance ISC include:
- Heavy Atom Effect: Introducing heavy atoms such as bromine or iodine on the BODIPY core significantly increases spin-orbit coupling, promoting triplet formation.
- Metal Coordination: Coordination with transition metal ions (e.g., Pt, Pd) can effectively modulate energy levels, improving triplet formation efficiency.
- Conjugation Extension and Energy Matching: Designing electron-acceptor structures conjugated with BODIPY facilitates excited-state electron transfer, activating the triplet channel.
These strategies enable BODIPY to serve as an efficient photosensitizer for PDT and photocatalytic energy conversion.
Controlling Singlet Oxygen Generation (for PDT)
Singlet oxygen (^1O₂) generation is one of the most critical mechanisms in photodynamic therapy. After photosensitizer excitation, ^1O₂ is produced through energy transfer, inducing oxidative damage to target cells. The efficiency of ^1O₂ generation in native BODIPY is limited, necessitating targeted optimization. Strategies to enhance singlet oxygen production include:
- Heavy Atom Modification: Similarly leveraging the heavy atom effect to increase triplet quantum yield, thereby boosting ^1O₂ generation.
- Conjugation System Expansion: Extending π-electron delocalization prolongs excited-state lifetime, facilitating more effective energy transfer.
- Push-Pull Electronic Modification: Introducing strong electron-donating or -withdrawing groups at meso or β positions promotes intramolecular charge transfer (ICT), enhancing excited-state energy utilization.
- Nanocarrier Integration: Embedding BODIPY into nanostructures (e.g., gold nanoparticles, polymer capsules) enhances the local light field and stabilizes the excited state, improving ROS generation efficiency.
Through these approaches, BODIPY derivatives have emerged as strong alternatives to traditional porphyrin-based photosensitizers in PDT.
Designing Photoswitchable or Photoactivatable BODIPY Probes
Beyond optimizing triplet states and singlet oxygen, photoresponsive BODIPY probes are increasingly studied. Photoswitchable and photoactivatable probes undergo reversible or irreversible structural or emission changes under specific wavelengths, finding applications in super-resolution imaging, drug release, and molecular machines. Common strategies to achieve photoresponsive BODIPY include:
- Coupling with Photosensitive Units: Linking BODIPY with photochromic groups such as azobenzenes or diarylethenes for light-induced reversible switching.
- Designing Photocages: Introducing photolabile protecting groups on BODIPY to release fluorescence or drugs upon irradiation at specific wavelengths.
- Constructing Energy Transfer Systems: Using FRET or PET mechanisms to achieve controllable on/off fluorescence signals under light exposure.
These probes offer spatiotemporal control, enabling precise molecular manipulation in complex biological environments, advancing BODIPY applications in neuroscience, targeted drug delivery, and super-resolution microscopy.
Improving Chemical Stability and Photostability of BODIPY
Like many organic fluorescent dyes, BODIPY may face photobleaching and chemical degradation under prolonged light exposure, complex biological environments, or extreme storage conditions. These issues not only reduce signal stability but may also bias experimental data, limiting reliability in clinical or industrial applications. Improving chemical and photostability is thus key to enhancing practical value and extending the dye's lifespan.
Common Degradation Pathways in Light and Biological Media
During applications, native BODIPY is mainly subject to:
- Photobleaching: Under strong or continuous excitation, interaction between the excited state and molecular oxygen can produce free radicals or singlet oxygen, damaging the molecular core and gradually diminishing fluorescence.
- Oxidative Degradation: BODIPY is prone to photooxidation in oxygen-containing environments, especially derivatives with higher triplet yields that generate ROS and self-degrade.
- Polar Environment-Induced Disassembly: In highly polar or aqueous solutions, intermolecular interactions can increase non-radiative decay and structural instability.
- Metabolic and Enzymatic Degradation in Biological Environments: Esterases or oxidases in cells or in vivo can affect BODIPY derivatives, leading to chemical structure breakdown.
Core Shielding via Substituents or Encapsulation
To enhance chemical and photostability, researchers have developed molecular shielding and encapsulation strategies:
- Introducing Sterically Bulky Substituents: Adding large aromatic groups at meso or β positions forms a spatial protective layer, reducing ROS or radical attacks on the core.
- Fluorination and Heteroatom Modification: Fluorine substitution on BODIPY can improve bond stability and reduce photodegradation.
- Nanocarrier Encapsulation: Embedding BODIPY in liposomes, polymer nanoparticles, or silica shells improves water solubility and isolates molecular oxygen, extending dye lifespan.
- Protein or Polymer Binding: Conjugation with albumin, PEG, or other polymers provides an additional stability barrier and enhances circulation time in biological systems.
These strategies allow BODIPY to maintain strong fluorescence output during prolonged excitation and in complex biological environments, suitable for continuous imaging and long-term experiments.
Design Approaches for Long-Term Storage and In Vivo Use
In addition to immediate application stability, long-term storage and in vivo use require special attention:
- Molecular Design: Introducing electron-donating or -withdrawing groups on the BODIPY core adjusts energy levels and reduces excited-state energy loss, lowering photodegradation.
- Optimized Storage Conditions: Avoiding light, high temperature, and strong oxidative environments—commonly storing BODIPY in dark, low-temperature, and inert gas-protected conditions—slows spontaneous degradation.
- Biological Application Optimization: For in vivo use, ensuring dye stability under physiological conditions is critical. Strategies include PEGylation to increase circulation time, surface functionalization for immune evasion, and nanoplatform loading to resist enzymatic degradation.
- Considerations for PDT: For photodynamic therapy, a balance must be achieved between high ROS generation and molecular stability. Structural modifications allow BODIPY to generate ROS efficiently while maintaining reasonable stability for sustained therapeutic action.
Through these multi-dimensional strategies, BODIPY dyes can maintain stable optical performance during storage, transport, and in vivo use, facilitating their translation from laboratory research to clinical and industrial applications.
BODIPY Dye Structural Design for Balanced Performance
The application performance of BODIPY dyes depends on multiple factors, including fluorescence efficiency, photochemical activity, stability, and biocompatibility. However, these properties often compete with each other: modifications that enhance one aspect may compromise another. Therefore, rational molecular design and site-specific modifications are key to optimizing the overall performance of BODIPY. In molecular engineering, researchers need to find a balance among brightness, reactivity, solubility, and stability to meet specific application requirements.
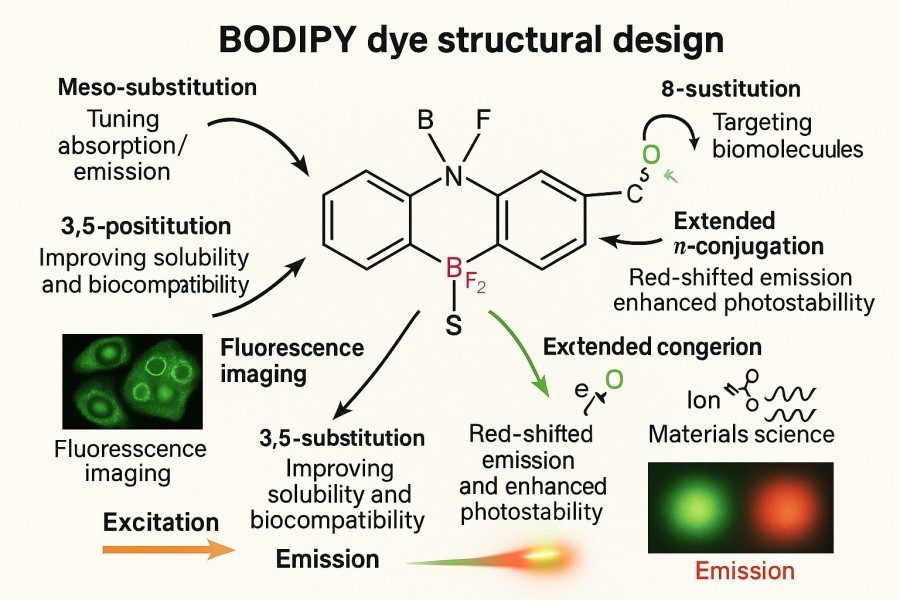 Fig. 2. BODIPY dye structural design (BOC Sciences Authorized).
Fig. 2. BODIPY dye structural design (BOC Sciences Authorized).
Trade-offs Between Brightness, Reactivity, and Solubility
In the molecular design of BODIPY derivatives, researchers often face trade-offs among brightness, reactivity, and solubility:
- Brightness vs. Reactivity: High fluorescence quantum yield usually requires minimizing non-radiative decay pathways. However, introducing heavy atoms to enhance triplet efficiency and photosensitizing activity can reduce fluorescence brightness. Therefore, BODIPY designed for imaging differs significantly in molecular strategy from BODIPY designed for photodynamic therapy.
- Brightness vs. Solubility: Large conjugated groups may be introduced to enhance fluorescence brightness, but these can lead to aggregation in aqueous systems, reducing effective signal output. Conversely, hydrophilic modifications (e.g., sulfonate groups, PEG chains) improve solubility but may alter electronic structure and lower fluorescence efficiency.
- Reactivity vs. Stability: Modifying BODIPY to enhance photodynamic or photoresponsive activity may alter energy levels, making the dye more prone to degradation and reducing stability.
Role of Substituent Position (meso-, α-, β-sites)
The BODIPY core consists of a boron-dipyrromethene structure, and substituents at different positions—meso, α, β—significantly affect molecular performance:
- meso position (8-position) modification: This site is highly sensitive for spectral tuning. Introducing aromatic or push/pull groups can significantly affect absorption and emission wavelengths while altering planarity and conjugation. Commonly used to red-shift emission or enhance charge transfer characteristics.
- α-positions (1,7-positions) modification: Significantly influences fluorescence quantum yield. Introducing electron-withdrawing groups often increases non-radiative decay, lowering fluorescence efficiency, but in photosensitizer design, this can promote triplet formation and singlet oxygen generation.
- β-positions (2,6-positions) modification: Strongly affects conjugation, serving as key sites for tuning fluorescence lifetime and emission wavelength. Bulky substituents can increase steric hindrance, preventing aggregation in aqueous systems and enhancing photostability.
- Boron center modification: Replacing BF₂ with other ligands (e.g., B-OR₂) can adjust electronic structure and chemical stability but may reduce ease of synthesis and storage.
Influence of Electron-Donating and Withdrawing Groups
BODIPY's optical and photochemical properties strongly depend on its electronic structure. Introducing electron-donating (EDG) and electron-withdrawing groups (EWG) is a core strategy for performance tuning:
- Electron-Donating Groups (EDG): Typical groups like methoxy or amino increase electron density, facilitating excited-state energy transfer to the emissive state, enhancing fluorescence brightness. Excessive donor strength may shorten excited-state lifetime, hindering triplet formation.
- Electron-Withdrawing Groups (EWG): Groups such as cyano, nitro, or carboxyl enhance intramolecular charge transfer (ICT) and prolong excited-state lifetime, benefiting singlet oxygen generation in PDT. However, this often reduces fluorescence quantum yield, making them more suitable for photochemical applications.
- Donor-Acceptor Architecture: Combining EDG and EWG at different positions enables ICT, achieving red-shifted emission, large Stokes shifts, and flexible photoresponsive behavior. This design is commonly used for near-infrared BODIPY and photoswitchable probes.
Collaborate with Us for Customized BODIPY Dyes and Technical Support
Different experimental systems and application scenarios require highly customized BODIPY dyes in terms of spectral properties, solubility, functional modifications, and conjugation strategies. Leveraging extensive experience in fluorescence chemistry, BOC Sciences provides full technical support from BODIPY design and structural optimization to functional modification and conjugation, helping clients overcome experimental challenges and develop efficient, reliable fluorescent probes. Our goal is to enhance dye performance and optimize experimental outcomes through tailored solutions, providing solid technical support for cutting-edge research and industrial applications.
Custom BODIPY Design and Synthesis
- Precisely design dye molecular structures according to client needs, including excitation/emission wavelength tuning, photostability optimization, and quantum yield enhancement.
- Offer multidimensional structural optimization strategies to balance spectral properties and solubility, improving usability in complex systems.
- Provide professional guidance for various experimental conditions to ensure optimal performance in in vitro imaging, live-cell labeling, and materials research.
Dye Functional Modification and Structural Optimization
- Support functional modifications such as introducing reactive groups, hydrophilic/hydrophobic tuning, or multicolor fluorescence labels to meet diverse research needs.
- Provide molecular design strategies to enhance dye stability and selectivity in biological systems or industrial materials.
- Enable targeted modification for applications in targeted labeling, environment-responsive fluorescence, or multifunctional probe development.
Conjugation and Labeling Technical Support
- Provide efficient conjugation solutions for proteins, nucleic acids, small molecules, and nanomaterials, optimizing product purity and labeling efficiency.
- Offer guidance on conjugation conditions and reaction monitoring for different molecular systems to ensure reproducibility and reliability.
- Support experimental design and data analysis to help clients efficiently complete labeling and application development.
Multifunctional Fluorescent Probe Development
- Assist in developing high-performance composite probes with imaging, tracking, and quantification functions for complex research and industrial applications.
- Provide probe performance evaluation, optimization, and application strategies to ensure stable and reliable use in cells, tissues, or material systems.
- Support end-to-end services from concept design to application implementation, accelerating the development and innovation of fluorescent tools.
Do You Need A Consultation?
BOC Sciences integrates cutting-edge fluorescence technologies to accelerate your research, driving next-generation solutions for drug discovery and diagnostics.
Transform Your Studies with Cutting-Edge Fluorescent Products
| Catalog | Name | CAS | Inquiry |
|---|---|---|---|
| F01-0277 | BDY TMR-X, SE | 217190-15-3 | Bulk Inquiry |
| F01-0153 | Pyrromethene 556 | 121461-69-6 | Bulk Inquiry |
| F01-0161 | BODIPY 558/568 C12 | 158757-84-7 | Bulk Inquiry |
| F01-0128 | 3,5-distyryl-BODIPYs | 1238620-18-2 | Bulk Inquiry |
| F01-0231 | BDP FL-PEG5-azide | 2093197-91-0 | Bulk Inquiry |
| F01-0228 | BDP 581/591 maleimide | 2183473-29-0 | Bulk Inquiry |
| F01-0230 | BDP 581/591 azide | 2183473-20-1 | Bulk Inquiry |
| F01-0236 | BDP 581/591 carboxylic acid | 480999-04-0 | Bulk Inquiry |
| F01-0275 | TechnoDye 498/505 | 105237-27-2 | Bulk Inquiry |
| F01-0007 | BDP 630/650 carboxylic acid | 2183512-02-7 | Bulk Inquiry |
High-Performance Fluorescent Tools for Your Research
- Alexa Fluor Bright, stable dyes for sensitive biosensing applications.
- Cyanine Versatile dyes used in biosensing and nucleic acid detection.
- Rhodamine Strong fluorescence, commonly used in protein and DNA sensing.
- Fluorescent Protein Used for live-cell imaging and real-time biosensing.
More About BODIPY Dyes
Online Inquiry

