Mastering BODIPY Fluorescent Labeling: Techniques, Applications, and Expert Tips
BODIPY (boron-dipyrromethene) dyes are gradually becoming the fluorescent tool of choice for researchers due to their high quantum yield, excellent photostability, narrow and tunable emission spectra, and extensive potential for molecular modification. Whether labeling proteins, peptides, lipids, or nucleic acids, BODIPY provides highly specific and bright signals, meeting the diverse needs of applications ranging from cellular imaging to high-throughput analysis. This article will explore BODIPY fluorescent labeling strategies, practical applications, and optimization tips, helping researchers overcome common challenges, enhance experimental efficiency and data reliability, and provide comprehensive technical guidance for both beginners and experienced scientists.
Introduction to BODIPY Fluorescent Labeling
In modern molecular imaging and bioanalytical research, the choice of fluorescent probes directly determines experimental sensitivity and accuracy. BODIPY fluorescent dyes have become the preferred choice for researchers and industry professionals due to their unique photophysical properties and highly tunable molecular structures. Compared to traditional fluorescent dyes, BODIPY offers high quantum yields, narrow and symmetric emission spectra, exceptional photostability, and a versatile molecular backbone, providing broad application potential in life sciences research, drug development, and materials science.
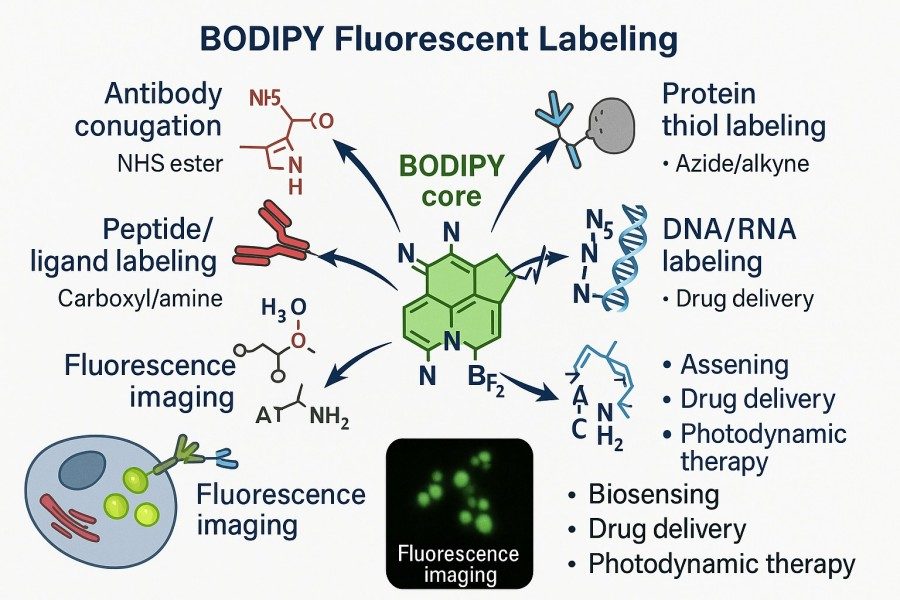 Fig. 1. BODIPY fluorescent labeling (BOC Sciences Authorized).
Fig. 1. BODIPY fluorescent labeling (BOC Sciences Authorized).
What Makes BODIPY Ideal for Fluorescent Tagging?
BODIPY molecules consist of a rigid pyrrole ring backbone coordinated with a boron atom. This unique chemical structure ensures both high brightness and stability in photophysical performance. Compared with commonly used dyes such as FITC or rhodamine, BODIPY demonstrates superior stability under varying pH, solvent polarity, and ionic strength conditions, making it ideal for labeling complex biological samples and long-term tracking. Furthermore, the BODIPY structure is highly modifiable. By introducing substituents on its backbone, researchers can flexibly tune its excitation and emission spectra, covering ranges from visible to near-infrared light. This property enables seamless integration into multicolor imaging and FRET experiments and broad applicability in high-precision techniques such as flow cytometry and confocal microscopy.
Spectral Characteristics and Photostability of BODIPY
The spectroscopic properties of BODIPY dyes greatly enhance their utility in research applications. These features effectively address common issues of conventional fluorescent dyes, such as signal decay, photobleaching, and background noise, especially in experiments requiring prolonged real-time imaging, dynamic monitoring, or high-resolution quantitative analysis.
- Narrow and strong excitation peaks, typically between 490–530 nm, reduce background interference.
- Symmetric and narrow emission peaks provide high signal resolution, suitable for multichannel detection.
- Exceptional photostability maintains fluorescence under prolonged excitation, preventing photobleaching from affecting experimental data.
- High molar extinction coefficients and quantum yields ensure signal brightness and detection sensitivity.
Use Cases Across Life Sciences and Material Research
Thanks to its flexible molecular modification potential and outstanding optical performance, BODIPY has become a core fluorescent tool across interdisciplinary research, providing scientists and engineers opportunities to surpass traditional methodological limitations.
- Life Sciences: Studies on cell membrane dynamics, protein localization, lipid metabolism tracking.
- Drug Development: Evaluation of targeted drug delivery systems, receptor-ligand interaction studies.
- Materials Science: Development of organic light-emitting diodes (OLEDs), photodynamic therapy (PDT) photosensitizers, and optoelectronic conversion materials.
Strategies for Efficient BODIPY Labeling
Efficient fluorescent labeling relies not only on probe performance but also on well-designed experimental strategies. For BODIPY dyes, various functional groups can be introduced via chemical modification of the backbone to achieve efficient conjugation with different biomolecules. Researchers should consider the conjugation method (covalent or non-covalent), target molecule type, and experimental conditions. Proper labeling strategies enhance signal specificity while minimizing non-specific binding and fluorescence quenching.
Covalent vs. Non-Covalent Labeling Methods
- Covalent Labeling: Covalent labeling uses reactive groups on BODIPY derivatives to chemically bind to amino, thiol, or carboxyl groups on target molecules, forming stable covalent bonds. This method ensures long-term fluorescence stability, ideal for experiments requiring prolonged tracking, such as protein localization, nucleic acid probe construction, or long-term intracellular imaging. Covalent labeling also provides reliable quantification, supporting precise analyses such as protein-ligand binding studies.
- Non-Covalent Labeling: Non-covalent labeling relies on hydrophobic interactions, electrostatic forces, or π-π stacking to temporarily associate BODIPY probes with target molecules. This approach is simple, rapid, and suitable for dynamic or transient binding studies, such as membrane structure imaging, lipid droplet labeling, or nanoparticle characterization. The reversible nature of non-covalent binding offers flexibility for studying molecular interactions while minimizing interference with target molecule function, facilitating experimental adjustment and optimization.
Functional Groups for Bioconjugation (NHS, Maleimide, Click Chemistry)
- NHS Ester (N-hydroxysuccinimide ester): The NHS ester in BODIPY dyes is a widely used reactive group that rapidly forms covalent bonds with primary amines on proteins or peptides, such as lysine side chains or N-terminal amino groups. This reaction proceeds under mild conditions and typically achieves high labeling efficiency, making it common in protein and peptide modifications. Care must be taken to avoid buffers containing free amines, which may react non-specifically with the NHS ester and reduce labeling efficiency.
- Maleimide: Maleimide groups are highly selective for thiols and commonly used for site-specific modification of cysteine residues. Given the limited number of thiols in proteins, this strategy enables precise protein labeling with minimal interference from other functional groups, offering advantages in high-specificity protein labeling and structure-function studies. In practice, proteins may need engineering to provide sufficient thiol sites for optimal labeling efficiency.
- Click Chemistry (Azide-Alkyne Cycloaddition): Click chemistry is a rapidly developing bioconjugation technique that typically employs cycloaddition reactions between azide and alkyne groups for efficient conjugation. This reaction is highly specific, unaffected by other functional groups, fast, and high-yielding, making it suitable for labeling in complex biological systems, such as in vivo probe labeling and precise molecular modifications. Click chemistry provides flexible strategies for reliable and controllable BODIPY labeling in complex environments.
Target Molecules: Proteins, Peptides, Lipids, Nucleic Acids
- Proteins are among the most common targets for BODIPY labeling. Using reactive groups such as NHS esters or maleimides, BODIPY can form stable covalent bonds with amino or thiol groups on proteins, achieving high-specificity labeling. These modifications help study protein folding and conformational changes and analyze protein-protein interactions and dynamic localization, making BODIPY indispensable in protein function research and imaging experiments.
- Peptides can also be targeted for BODIPY labeling, especially for studying cell-penetrating peptides, signal peptides, or functional short peptides' intracellular transport and localization. Selective modification at the peptide's C- or N-terminus allows efficient fluorescent labeling without compromising biological activity, enabling real-time monitoring in vitro or within cellular systems.
- Lipids, due to their hydrophobic nature, naturally interact with the hydrophobic BODIPY backbone, allowing high-fidelity labeling of membrane structures and lipid droplets. This non-covalent or weakly covalent binding gives BODIPY a unique advantage in membrane dynamics studies, lipid metabolism tracking, and cell membrane signaling pathway analysis, while multicolor imaging enables high-resolution observation of cellular membranes and microstructures.
- Nucleic acids represent another important target for BODIPY labeling. Through click chemistry or phosphate modifications, BODIPY can bind DNA or RNA with high specificity. This strategy is widely used in real-time nucleic acid imaging, gene expression analysis, and hybridization detection, providing clear signals and maintaining sensitivity and specificity in high-throughput assays, offering reliable tools for molecular biology and genomics research.
Fluorescence Services Powered by BOC Sciences
| Solutions | Capabilities |
|---|---|
| Bioconjugation | Enabled with high-purity BODIPY dyes and optimized conjugation strategies for precise and stable biomolecule labeling. |
| Drug Delivery | Supported by custom-designed BODIPY probes for monitoring distribution, release kinetics, and therapeutic targeting. |
| Fluorescence Imaging | Achieved with photostable, water-soluble BODIPY derivatives ensuring bright, uniform, and high-resolution visualization. |
| Flow Cytometry | Enhanced by high-brightness BODIPY dyes with uniform signal distribution for reliable cell population analysis. |
| Cell Imaging | Advanced through cell-permeable BODIPY probes enabling real-time tracking of organelles and dynamic cellular processes. |
Water-Soluble and Cell-Permeable BODIPY Derivatives
The original BODIPY backbone is highly hydrophobic, which leads to low solubility, uneven distribution, and potential aggregation in aqueous biological environments, limiting its efficiency in cellular imaging and in vivo applications. Therefore, developing water-soluble and cell-permeable BODIPY derivatives has become a key focus for researchers. By optimizing BODIPY's hydrophilicity and biocompatibility through molecular design, its applicability in life science research can be significantly expanded while retaining its excellent optical properties.
Structural Modifications of BODIPY for Aqueous Compatibility
To enhance BODIPY stability in aqueous solutions, researchers typically introduce hydrophilic groups into the molecular backbone. For example, sulfonic acid groups increase the overall negative charge, improving solubility in water; quaternary ammonium groups enhance intracellular distribution through positive charge interactions with biomolecules; and polyethylene glycol (PEG) chains not only greatly increase water solubility but also improve biocompatibility and reduce the risk of non-specific binding. These modification strategies maintain the core fluorescent performance of BODIPY while promoting uniform distribution in cellular or tissue environments, making them suitable for a wide range of in vitro and in vivo imaging experiments.
Application of BODIPY in Live-Cell Imaging
Water-soluble and cell-permeable BODIPY probes are highly valuable for live-cell imaging. Their ability to distribute evenly in aqueous environments and penetrate cell membranes allows real-time tracking of cellular metabolic activity, observation of organelle dynamics such as mitochondria and endoplasmic reticulum, and support for multicolor confocal imaging, enabling high-resolution analysis of intracellular structure and function. Additionally, these probes typically exhibit low cytotoxicity and good photostability, making long-term dynamic observation possible and providing reliable tools for cell biology research.
Comparison of Water-Soluble vs. Hydrophobic Dyes
The greatest advantage of water-soluble BODIPY dyes is their uniform and stable signal, making them ideal for intracellular imaging and studies in aqueous systems. However, for experiments targeting membrane structures, lipid droplets, or hydrophobic microenvironments, hydrophobic BODIPY remains invaluable. Its hydrophobic backbone facilitates insertion into lipid bilayers or hydrophobic microdomains, enabling high-fidelity imaging of specific structures. Nonetheless, hydrophobic dyes are prone to aggregation and uneven distribution, so experimental design must carefully balance the application scenario and labeling efficiency or combine with hydrophilic modifications to improve performance.
Best Practices for Fluorescent Labeling Workflows
Efficient and stable BODIPY labeling relies not only on the dye itself but also on optimizing the entire labeling workflow. From sample preparation to reaction conditions and post-treatment, each step can influence fluorescence intensity, specificity, and uniformity. Mastering a scientifically designed workflow helps avoid common issues such as signal loss, non-specific binding, or probe aggregation, improving reproducibility and data reliability.
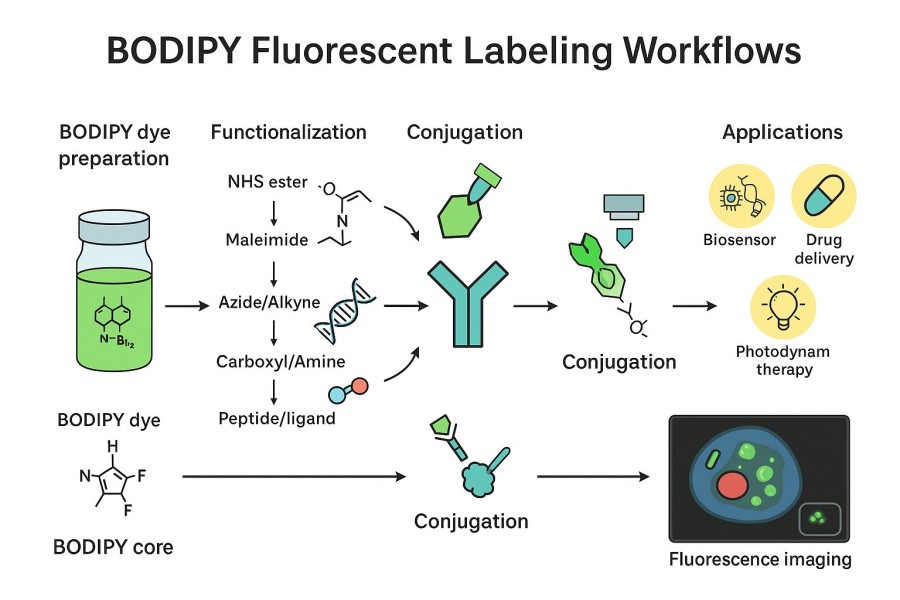 Fig. 2. Fluorescent labeling workflows (BOC Sciences Authorized).
Fig. 2. Fluorescent labeling workflows (BOC Sciences Authorized).
Sample Preparation and Buffer Conditions
Sample preparation is the first and crucial step for ensuring high-quality fluorescence signals. In protein or peptide labeling, impurities that may interfere with conjugation—such as free amines, thiol inhibitors, or reducing agents—should be removed as they can react non-specifically with BODIPY's reactive groups. Buffer selection is equally important; phosphate-buffered saline (PBS) or HEPES buffers without free amines are recommended, with pH controlled within the dye's optimal reaction range to preserve both reactive group activity and target molecule integrity. For cell or tissue samples, cleanliness and compatibility with subsequent staining and imaging are essential to minimize background signals.
Concentration Optimization and Incubation Times
BODIPY labeling efficiency is closely related to dye concentration and reaction time. Too low a concentration may lead to insufficient labeling and weak signals, whereas too high a concentration can cause aggregation or non-specific binding, reducing signal specificity. Gradient optimization experiments should be performed to determine the optimal dye concentration and reaction time. For protein labeling, small-scale trials can help adjust concentration while monitoring fluorescence intensity and distribution uniformity. For live-cell labeling, dye permeability and metabolic clearance must also be considered, with incubation times optimized to achieve efficient labeling without affecting cell viability.
Preventing Aggregation and Non-Specific Binding
During labeling, BODIPY molecules may aggregate or bind non-specifically, reducing labeling efficiency and introducing background signals. Multiple strategies can mitigate these issues: first, use water-soluble or partially hydrophilic BODIPY derivatives to improve dispersion in aqueous solutions; second, adding surfactants or protein blockers (e.g., BSA) can reduce non-specific adsorption; finally, controlling reaction temperature, ionic strength, and pH helps maintain dye and target molecule stability. These approaches ensure uniform and reproducible labeling signals, providing reliable data for microscopy or analytical studies.
Challenges and Troubleshooting in BODIPY Labeling
Although BODIPY dyes demonstrate excellent performance in fluorescent labeling, practical applications may encounter challenges. Signal reduction, spectral overlap, and low labeling efficiency are common issues that can affect experimental accuracy and reproducibility. By systematically analyzing these challenges and applying targeted strategies, researchers can optimize labeling workflows and improve experimental success rates.
Signal Loss and Quenching Issues
Signal loss or quenching is one of the most common problems in BODIPY labeling. It can result from dye aggregation, changes in solution conditions, or energy transfer with other molecules. For example, hydrophobic BODIPY tends to aggregate at high concentrations, leading to self-quenching; intracellular reductants or metal ions may also affect fluorescence performance. These issues can be addressed by using water-soluble derivatives or introducing hydrophilic modifications to reduce aggregation, and by optimizing dye concentration and reaction conditions to prevent non-specific quenching.
Spectral Overlap and Crosstalk
In multicolor imaging and FRET experiments, BODIPY dyes may overlap with the excitation or emission spectra of other fluorophores, causing crosstalk. Spectral overlap not only reduces signal resolution but also interferes with quantitative analysis. To minimize this, spectral matching should be considered during experimental design, selecting excitation and emission wavelength combinations with sufficient separation. Additional strategies such as filter optimization, line-scanning imaging, or spectral unmixing can further reduce crosstalk and enhance multicolor imaging specificity.
Low Labeling Efficiency or Poor Conjugation Yield
Low labeling efficiency or conjugation yield often arises from limited reactive groups on the target molecule, consumption of reactive groups by buffers or impurities, or inappropriate reaction conditions. To improve conjugation efficiency, ensure that the target molecule is purified to remove interfering substances; select appropriate functional groups and conjugation chemistry—NHS esters for amine modification, maleimides for thiol modification, or click chemistry for precise conjugation. Gradient optimization of dye concentration, reaction time, and temperature can significantly enhance labeling efficiency and yield, resulting in stable and bright fluorescent signals.
Selecting the Right BODIPY Dye for Your Application
Choosing the appropriate BODIPY dye for a specific experiment is critical to ensuring labeling effectiveness and experimental success. Different BODIPY dyes vary in spectral properties, chemical modifications, cell permeability, and suitability for applications. Therefore, researchers must consider fluorescence spectral matching, the target molecule, and the detection system when designing experiments to achieve high-sensitivity, high-specificity imaging or analysis.
Fluorescence Spectra Matching with Detection Systems
In multicolor imaging, flow cytometry, or FRET experiments, the excitation and emission spectra of BODIPY dyes must be compatible with the detection system. When selecting a dye, ensure that its excitation peak can be efficiently excited by the microscope or flow cytometer light source, and that the emission peak falls within the detector's spectral range for effective capture. Additionally, in multicolor experiments, spectral separation between dyes should be sufficient to minimize signal crosstalk and interference. This strategy not only improves signal resolution but also ensures the accuracy of multichannel imaging and quantitative analysis.
Choosing BODIPY for FRET, Flow Cytometry, or Confocal Imaging
Different experimental applications impose distinct performance requirements on BODIPY dyes. In FRET experiments, selecting dyes with narrow emission spectra and high photostability can enhance energy transfer efficiency and signal detection sensitivity. In flow cytometry, dyes should exhibit high brightness and uniform distribution to ensure reliable analysis of cell populations. For confocal imaging, cell permeability and photostability are key factors, ensuring even intracellular distribution and stable signals during prolonged imaging. Selecting the most suitable BODIPY type according to the experimental goal can significantly improve data quality and analytical accuracy.
Commercially Available vs. Custom-Designed BODIPY Probes
When selecting a BODIPY dye, researchers must also consider whether to use commercially available dyes or commission custom-designed derivatives. Commercial dyes typically offer established synthesis routes, reliable quality control, and ready-to-use specifications, making them suitable for routine experiments and rapid validation. Custom BODIPY probes allow molecular backbone modifications or functional group designs tailored to specific target molecules or imaging needs, enabling high-specificity conjugation, optimized spectral properties, or improved cell permeability. In complex or high-demand experiments, custom dyes provide advantages unattainable with off-the-shelf products, meeting the requirements of advanced research and precision imaging.
Work with Us for High-Quality BODIPY Dyes and Labeling Solutions
Choosing a reliable supplier and technical support is essential in BODIPY fluorescent labeling research and applications. BOC Sciences, with extensive experience in fluorescent chemistry and a comprehensive service system, provides high-quality BODIPY dyes and complete labeling solutions, supporting experimental success and enhancing data reliability.
High-Purity BODIPY Dye Synthesis for Bioconjugation
- Laboratory synthesis and custom compound services for high-quality BODIPY dyes, meeting diverse structural and functional needs.
- Supports core backbone modifications, hydrophilic/hydrophobic tuning, and incorporation of specialized functional groups to optimize spectral properties and biocompatibility.
- Strict control of purity and batch-to-batch consistency ensures dye stability in subsequent labeling and imaging.
- Scalable to pilot or industrial production according to client requirements, balancing research flexibility with large-scale application.
Custom BODIPY Probe Design and Conjugation Services
- Molecular backbone modifications and functional design to optimize fluorescence properties, cell permeability, and water solubility based on experimental requirements.
- Supports site-specific labeling of target biomolecules (proteins, peptides, lipids, nucleic acids) to enhance labeling specificity and experimental reproducibility.
- Probes can be designed for FRET, multicolor confocal imaging, flow cytometry, and live-cell tracking applications.
- Flexible reaction strategies available, including NHS ester, maleimide, and click chemistry conjugation, to meet diverse experimental needs.
Analytical Validation and Purity Control
- Each batch of BODIPY dye is analyzed by HPLC, MS, and NMR to ensure molecular structure accuracy.
- Purity reports and fluorescence performance validation are provided to guarantee consistency in practical applications.
- Performance evaluations are conducted for water-soluble, hydrophobic, and functionalized derivatives to ensure reliable distribution and stable signals in biological samples.
- Custom quality control programs can be provided according to client requirements, meeting research and industrial standards.
Technical Support for Assay Development and Scale-Up
- Provides experimental design guidance, including dye selection, labeling strategies, buffer optimization, and fluorescence detection parameter adjustment.
- Supports technical transfer and scale-up of labeling workflows, enabling smooth transition from small-scale trials to large-scale production.
- Offers professional solutions to common issues such as signal quenching, non-specific binding, and low labeling efficiency.
- Provides client training and technical guidance to ensure research teams quickly master BODIPY labeling techniques and optimize experimental workflows.
Do You Need A Consultation?
BOC Sciences integrates cutting-edge fluorescence technologies to accelerate your research, driving next-generation solutions for drug discovery and diagnostics.
Transform Your Studies with Cutting-Edge Fluorescent Products
| Catalog | Name | CAS | Inquiry |
|---|---|---|---|
| F01-0154 | BODIPY FL acid | 126250-45-1 | Bulk Inquiry |
| F01-0159 | Pyrromethene 580 | 151486-56-5 | Bulk Inquiry |
| F01-0160 | Pyrromethene 650 | 157410-23-6 | Bulk Inquiry |
| F01-0043 | CRANAD 28 | 1623747-97-6 | Bulk Inquiry |
| F01-0042 | MitoPerOx | 1392820-50-6 | Bulk Inquiry |
| F01-0129 | NIR-BODIPYs-free acid | 1996627-88-3 | Bulk Inquiry |
| F01-0224 | BDP FL-PEG4-TCO | 2183473-16-5 | Bulk Inquiry |
| F01-0232 | BDP FL-PEG5-propargyl | 2093197-93-2 | Bulk Inquiry |
| F01-0016 | BDP R6G carboxylic acid | 174881-57-3 | Bulk Inquiry |
| F01-0017 | BDP R6G maleimide | 2183473-32-5 | Bulk Inquiry |
High-Performance Fluorescent Tools for Your Research
- Alexa Fluor Bright, stable dyes for sensitive biosensing applications.
- Cyanine Versatile dyes used in biosensing and nucleic acid detection.
- Rhodamine Strong fluorescence, commonly used in protein and DNA sensing.
- Fluorescent Protein Used for live-cell imaging and real-time biosensing.
More About BODIPY Dyes
Online Inquiry

