Understanding the Excitation and Emission Properties of BODIPY Dyes: A Guide for Optimal Fluorescent Probe Selection
BODIPY (Boron-Dipyrromethene) dyes have become one of the most sought-after fluorescent tools for researchers due to their high fluorescence quantum yields, excellent photostability, and tunable spectral properties. However, different BODIPY derivatives exhibit significant variations in excitation and emission characteristics, and improper selection may result in weak signals, spectral crosstalk, or experimental failure. Therefore, a thorough understanding of BODIPY's optical principles, excitation/emission profiles, and structure–function relationships is essential for efficient experimental design and fluorescent probe optimization. This guide systematically explores the core structure, spectral properties, structural modification effects, and application-matching strategies of BODIPY dyes, helping researchers select the most suitable fluorescent probes for life sciences, materials science, and energy research.
Introduction to BODIPY Fluorophores
Fluorescent dyes play a crucial role in life sciences, materials science, and energy research. Among them, BODIPY dyes have attracted significant attention due to their unique structure and superior optical properties. Compared with conventional fluorescent molecules, BODIPY offers high fluorescence quantum yields, strong photostability, and flexible spectral tunability, ensuring excellent performance even in complex experimental environments.
What Is BODIPY? Overview of Its Core Structure
The core structure of BODIPY consists of dipyrromethene coordinated with boron trifluoride (BF₂). This unique planar and rigid scaffold not only minimizes non-radiative energy loss in the excited state, enabling high fluorescence efficiency and strong signal output, but also provides multiple sites for molecular modification. By introducing electron-donating or electron-withdrawing groups at different positions, the spectral properties can be flexibly tuned. For example, incorporating aromatic groups at positions 3 and 5 extends the conjugation system, achieving red-shifted emission spectra, while adding hydrophilic groups at position 8 improves solubility and biocompatibility. These engineered derivatives meet diverse application needs in cell imaging, optoelectronic devices, and energy research. The highly customizable scaffold is the foundation of BODIPY's high performance and a key reason for its prominence in fluorescent probe design.
 Fig. 1. Structure of BODIPY and it's derivatives (BOC Sciences Authorized).
Fig. 1. Structure of BODIPY and it's derivatives (BOC Sciences Authorized).
Why BODIPY Is Popular in Fluorescent Applications
The widespread adoption of BODIPY dyes stems from several advantages, enabling excellent performance in single-color imaging as well as multi-labeling, flow cytometry, FRET experiments, and advanced optical systems:
- High Photostability: Maintains strong and stable fluorescence under continuous excitation, suitable for real-time or long-term imaging.
- Tunable Spectra: Molecular modifications allow excitation/emission wavelengths to span 500–800 nm, covering applications from shallow to deep-tissue imaging.
- Narrow and Symmetric Emission Peaks: Minimizes spectral crosstalk, facilitating combination with other dyes in multi-labeling experiments.
- High Quantum Yield: Provides bright signals even at low concentrations, enhancing detection sensitivity.
- Strong Chemical Stability: Maintains structural integrity in acidic, basic, or organic solvent conditions, broadening the scope of applications.
Typical Use Cases Across Biology, Materials, and Energy Fields
BODIPY dyes have a wide range of applications, extending from basic research to industrial use. These cross-disciplinary applications demonstrate that BODIPY is not merely an experimental tool but a strategic molecule driving new materials and energy technologies.
- Biology: Cell imaging, flow cytometry, FRET probes, protein labeling.
- Materials Science: Organic light-emitting diodes (OLEDs), photosensitive materials, nanoscale probes.
- Energy Research: Photosensitive dyes, light-harvesting materials for solar cells.
Basic Principles of Fluorescence: Excitation and Emission
Understanding the basic principles of fluorescence is fundamental to successful probe research and applications. Fluorescence is essentially a photophysical phenomenon, where a molecule absorbs a photon to reach an excited state and then releases a lower-energy photon via radiative transition. The spectral characteristics of different fluorescent molecules largely determine their suitability in specific experimental settings, and BODIPY, as a high-performance dye, warrants in-depth understanding of its excitation and emission properties.
Key Definitions: Excitation Wavelength, Emission Wavelength, and Stokes Shift
Three core parameters must be clarified when studying fluorescent molecules, serving as essential criteria for probe selection:
- Excitation Wavelength (λex): The range of wavelengths absorbed to excite the molecule, dictating the choice of light source. For BODIPY, excitation typically falls within the visible range, compatible with common light sources.
- Emission Wavelength (λem): The wavelength of photons released as the molecule returns to the ground state, representing the detectable fluorescence signal. BODIPY emission peaks are usually narrow and clean, allowing clear distinction in multi-labeling experiments.
- Stokes Shift: The difference between excitation and emission wavelengths, indicating energy loss. A larger Stokes shift reduces background interference. BODIPY generally has a small Stokes shift, which ensures high signal brightness but requires precise filter matching in multi-color experiments.
How Fluorescence Works in BODIPY Dyes?
Fluorescence relies on molecular electronic transitions. Upon absorbing photons of specific wavelengths, electrons in BODIPY molecules are promoted from the ground state to the excited state. Some energy is dissipated as molecular vibrations or heat, a process known as non-radiative relaxation. Due to BODIPY's rigid planar scaffold, non-radiative energy loss is minimized, enabling near-ideal photon emission efficiency. This structural feature underlies BODIPY's high quantum yield and bright fluorescence signals.
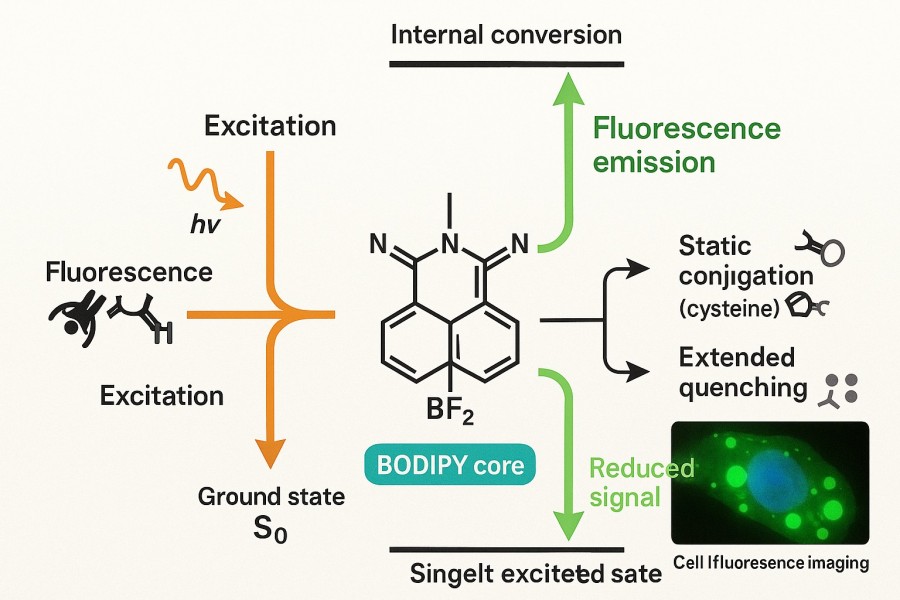 Fig. 2. BODIPY mechanism in fluorescence (BOC Sciences Authorized).
Fig. 2. BODIPY mechanism in fluorescence (BOC Sciences Authorized).
Moreover, BODIPY exhibits high spectral stability in solution, with minimal fluorescence quenching or energy transfer, making it ideal for single-molecule imaging, long-term cell tracking, and real-time kinetic studies. Unlike some traditional dyes prone to photobleaching, BODIPY maintains stable emission under continuous excitation, which is particularly valuable for extended observations.
Instrumentation and Spectral Matching Basics
Fluorescent probe performance depends not only on the dye's properties but also on experimental instrumentation. Light sources, filters, and detectors must be carefully matched to BODIPY's excitation/emission spectra; otherwise, even high-performance dyes may fail to achieve optimal results.
- Light Source Selection: Common sources include xenon lamps, mercury lamps, lasers, and LEDs. BODIPY excitation typically falls in the visible range, compatible with most sources. For high-resolution or multi-color experiments, lasers offer more stable excitation.
- Filter Matching: Filters selectively transmit excitation or emission light, ensuring signal purity. BODIPY's narrow emission peaks require carefully designed filter bandwidths to prevent excitation light leakage.
- Detector Sensitivity: Detectors include PMTs and CCD/CMOS cameras. For weak signal detection, high-sensitivity cooled CCD cameras better capture BODIPY fluorescence.
Improper optical system configuration can lead to signal loss, background interference, or spectral overlap, seriously compromising experimental reliability. Researchers should consider spectral matching during experimental design to fully leverage BODIPY probe advantages.
Tools and Techniques for Measuring Spectral Properties
Accurately measuring the excitation and emission properties of BODIPY dyes is critical for experimental design and fluorescent probe optimization. Spectral measurements not only help researchers understand the fundamental optical performance of dyes but also guide their applications in imaging, FRET, flow cytometry, and photosensitive material development. Common tools and techniques include spectrofluorometers, absorbance scans, quantum yield measurements, fluorescence lifetime analysis, and spectral database consultation. These methods provide a comprehensive evaluation of BODIPY optical properties, offering reliable data to support experiments.
Spectrofluorometry and Absorbance Scans
Spectrofluorometers are the primary tools for measuring the excitation and emission spectra of BODIPY dyes. By adjusting the excitation wavelength and recording the emission spectrum, researchers obtain complete spectral curves that reveal peak positions, bandwidths, and Stokes shifts. Combined with UV–Vis absorbance scans, the absorption properties and molar extinction coefficients of BODIPY can be analyzed to assess light absorption efficiency. Precise spectral measurements are essential for FRET design, multi-label experiments, and optical channel configuration.
Quantum Yield and Lifetime Analysis
Quantum yield (QY) and fluorescence lifetime are key indicators for evaluating BODIPY fluorescence efficiency and stability. Quantum yield reflects the efficiency of photon emission during relaxation from the excited state, with higher values indicating brighter signals and better signal-to-noise ratios. Fluorescence lifetime analysis reveals the excited-state dynamics of molecules, which can be used to distinguish dyes, detect environmental sensitivity, and design FRET experiments. Systematic measurement of BODIPY quantum yields and lifetimes allows researchers to optimize experimental conditions and select the most suitable fluorescent probes for specific applications.
Fluorescence Spectral Libraries and Databases
To efficiently screen suitable BODIPY dyes, researchers can utilize fluorescence spectral libraries and databases. These resources provide extensive data on excitation/emission spectra, quantum yields, lifetimes, and application examples, enabling rapid performance comparisons between dyes. Databases allow researchers to predict spectral compatibility before multi-color imaging or FRET experiments, reducing setup time and improving workflow efficiency. Some databases also offer structure-based spectral prediction functions, providing guidance for designing novel BODIPY derivatives.
Excitation and Emission Spectra of Common BODIPY Dyes
A notable advantage of BODIPY dyes is their spectral tunability via molecular modifications, covering a broad range from visible to near-infrared (NIR) wavelengths. This flexibility forms the basis for applications in multi-color imaging, energy transfer, and bioanalytical studies. Common BODIPY dyes include standard green-emitting derivatives, red/NIR-shifted derivatives, and specialized variants such as Aza-BODIPY, halogenated BODIPY, and π-extended BODIPY, each exhibiting distinct excitation and emission spectral characteristics.
Standard BODIPY (Green-Emitting): Excitation ~500 nm / Emission ~510–530 nm
Classic BODIPY derivatives typically have excitation around 500 nm and emission between 510–530 nm, producing bright green fluorescence. Key features include:
- High Fluorescence Quantum Yield: Often near 0.9–1.0, ensuring exceptional brightness in the visible range.
- Narrow Emission Bandwidth: Full width at half maximum (FWHM) generally<20 nm, providing high signal purity.
- Excellent Photostability: Resistant to photobleaching under strong illumination, suitable for long-term imaging.
Applications of standard BODIPY include cell membrane staining, intracellular small molecule tracking, and FRET donor labeling. Its green emission allows combination with red or far-red fluorophores for multi-color imaging while minimizing spectral overlap.
Red/NIR-Shifted BODIPYs (630–800 nm Range)
Extending the conjugation or introducing electron donor/acceptor groups on the BODIPY scaffold enables significant red-shifting of excitation and emission spectra. Red/NIR-emitting BODIPY features include:
- Excitation 600–650 nm, Emission 630–800 nm: Covering far-red to NIR regions.
- Enhanced Tissue Penetration: NIR light reduces autofluorescence and improves tissue penetration, ideal for in vivo small animal imaging.
- Reduced Phototoxicity: Longer wavelength excitation minimizes light-induced damage, suitable for real-time kinetic studies and deep-tissue imaging.
For example, NIR BODIPY probes show great potential in photodynamic therapy (PDT), photoacoustic imaging, and cancer-targeted diagnostics. Compared with traditional green BODIPY, red/NIR derivatives are more suitable for biological applications and can also meet the requirements of solar cell photosensitizers in energy research.
Aza-BODIPY, Halogenated, and π-Extended Variants
Beyond standard and red-shifted BODIPY, a variety of structural derivatives have been developed to expand spectral and functional versatility, providing researchers with greater experimental design flexibility:
- Aza-BODIPY: Incorporates nitrogen atoms into the BODIPY core, significantly altering electronic properties and shifting excitation/emission to the NIR region (650–800 nm). These dyes exhibit strong photostability and bright emission, ideal for deep tissue and in vivo imaging.
- Halogenated BODIPY: Incorporates Cl, Br, or I atoms to enhance intersystem crossing (ISC), increasing triplet-state yields. This property enables efficient singlet oxygen generation, making halogenated BODIPY valuable for photodynamic therapy (PDT) studies.
- π-Extended BODIPY: Adds additional aromatic groups to pyrrole or benzene rings, extending conjugation and red-shifting emission (typically 700–900 nm, potentially >1000 nm in NIR-II). These dyes exhibit enhanced light absorption and energy conversion efficiency, excelling in organic optoelectronics, photovoltaics, and photosensitizer research.
BODIPY Services by BOC Sciences
| Solutions | Capabilities |
|---|---|
| Lipid Staining | Enables precise visualization of cellular lipid structures with high signal stability and minimal background interference. |
| Cell Staining | Provides bright, photostable fluorescence for clear identification and tracking of diverse cell types. |
| Protein Staining | Facilitates efficient labeling of proteins with customizable conjugation for sensitive detection and multiplexing. |
| In Vivo Imaging | Supports deep-tissue, low-background imaging with optimized near-infrared emission and biocompatible design. |
| Cell Imaging | Delivers high-resolution, long-term fluorescence for detailed observation of live-cell dynamics. |
How Structural Modifications Affect Spectral Properties?
The excitation and emission spectra of BODIPY dyes are not solely determined by their core scaffold but can also be finely tuned through precise chemical modifications. Changes in molecular structure influence electron distribution, conjugation degree, and non-radiative energy loss, directly affecting fluorescence wavelength, brightness, and quantum yield. Understanding these principles is essential for designing fluorescent probes with specific spectral properties.
Substitution Effects on Wavelength Shift
Introducing substituents at different positions on the BODIPY scaffold can significantly alter spectral properties. For example, adding aromatic or electron donor/acceptor groups at positions 3 and 5 expands the π-conjugation system, causing red-shifts in emission. Substitutions at positions 1, 7, or 8 can modulate electron density and solubility, allowing fine-tuning of excitation and emission wavelengths. Small groups such as F, Cl, Br, or methyl can also affect molecular energy levels through electronic and steric effects, influencing Stokes shifts and fluorescence brightness. Through precise substituent design, researchers can achieve controlled emission across green, red, or near-infrared regions.
Expanding Conjugation to Achieve Red or NIR Emission
To obtain red or near-infrared (NIR) emitting BODIPY derivatives, conjugation expansion strategies are commonly used. Introducing aromatic groups, alkynes, or other conjugated units onto the pyrrole or benzene rings increases π-electron delocalization, reducing the energy gap and red-shifting emission. π-extension not only enables emission in the far-red or NIR region but also enhances light absorption efficiency and fluorescence brightness. These modifications are valuable for in vivo imaging, photodynamic therapy, and photosensitive material development, while providing flexible options for multi-color imaging experiments.
Solvent and pH Effects on Excitation/Emission Behavior
In addition to chemical modifications, external environmental factors can influence BODIPY spectral behavior. Solvent polarity, hydrogen bonding, and pH changes may induce slight shifts in excitation or emission peaks. For instance, in polar solvents, some BODIPY molecules may exhibit red-shifted emission or reduced fluorescence due to enhanced non-radiative relaxation via solvent interactions. pH-sensitive BODIPY derivatives may undergo structural rearrangements under different pH conditions, significantly altering excitation/emission spectra. These characteristics must be considered during experimental design and provide opportunities for developing environment-sensitive fluorescent probes.
Matching BODIPY Dyes to Your Application
Selecting the right BODIPY fluorescent probe is a critical step for successful experiments. Excitation/emission spectra, photostability, quantum yield, and chemical modifiability determine a dye's suitability for various research applications. Researchers should match dyes to the required signal intensity, sample environment, imaging depth, and multi-labeling needs to ensure high-quality imaging or detection results.
Choosing the Right Fluorophore for Imaging
In cell imaging, tissue sections, or in vivo experiments, BODIPY selection should consider excitation sources, emission detectors, and background autofluorescence. Green-emitting BODIPY is ideal for standard confocal imaging and single-cell studies, while red or NIR-emitting BODIPY offers stronger tissue penetration and lower background noise, suitable for in vivo or deep tissue imaging. Additionally, probe hydrophilicity, cell permeability, and potential non-specific binding should be considered to ensure efficient targeting and stable signal output.
Emission Profiles for FRET, Flow Cytometry, and Live-Cell Use
In FRET experiments, spectral overlap between donor and acceptor is critical. When used as a FRET donor, BODIPY emission must overlap the acceptor excitation for efficient energy transfer. Narrow emission peaks and strong photostability provide clear signals for accurate energy transfer calculation. In flow cytometry, narrow BODIPY emissions reduce spectral overlap when multiple probes are used, simplifying compensation. For live-cell imaging, BODIPY derivatives with high quantum yield, low phototoxicity, and good solubility enable long-term tracking while maintaining cellular physiology.
Considerations for Multiplexing and Minimal Crosstalk
In multi-label experiments, spectral overlap between probes can cause crosstalk and affect data accuracy. BODIPY dyes, with tunable excitation/emission wavelengths and narrow peaks, are ideal for multiplexing. Researchers can select well-separated BODIPY derivatives from spectral libraries and combine them with appropriate filters and optical channel setups to minimize crosstalk and maximize signal-to-noise ratios. Chemical modifications to control solubility, targeting, and photostability further enhance performance in complex biological systems, ensuring precise multi-color imaging.
Need a Specific Excitation/Emission Profile? Work with Us
In research and industrial applications, specific BODIPY excitation and emission wavelengths are often required for imaging, multiplexing, or photosensitive material development. BOC Sciences provides professional custom dye design and technical support to achieve desired optical properties. Services include:
Custom BODIPY Dye Design for Required Wavelengths
- Structural Optimization: Design optimal BODIPY molecules using substituents, conjugation extension, or scaffold modification to achieve target excitation/emission.
- Spectral Simulation and Prediction: Use advanced computational and spectral prediction tools to ensure designed molecules meet experimental optical requirements.
- Functional Customization: Introduce hydrophilic, cell-targeting, or biocompatible modifications to meet specific application needs.
Comprehensive BODIPY Conjugation Solutions
- Multiple Conjugation Strategies: Support amine, thiol, carboxyl, or click chemistry for effective coupling with proteins, antibodies, nucleic acids, or small molecules.
- Custom Functionalization: Add hydrophilic, cell-penetrating, or targeted ligands during conjugation to enhance biocompatibility and targeting.
- High Efficiency and Purity: Optimized conjugation and purification processes ensure structural integrity, stable optical performance, and complete QC data.
Technical Support for Spectral Matching and Application Setup
- Spectral Matching Consultation: Assist in selecting BODIPY dyes compatible with lasers, filters, and detectors to optimize signal strength and SNR.
- Application Optimization: Provide guidance for multi-color imaging, FRET design, flow cytometry, and live-cell experiments to reduce crosstalk and background interference.
- Problem Solving: Offer expert advice on photostability, solubility, and pH sensitivity issues to ensure smooth experimental execution.
Batch Synthesis, QC Data, and Application Consultation
- Custom Synthesis: Provide tailored synthesis services from milligram-scale experimental amounts to gram-scale industrial quantities.
- Strict Quality Control: Deliver high-resolution NMR, MS, and HPLC data to ensure each BODIPY batch meets design standards.
- Comprehensive Application Support: Offer dye storage, usage guidance, and data interpretation to maximize performance in research or industrial projects.
Do You Need A Consultation?
BOC Sciences integrates cutting-edge fluorescence technologies to accelerate your research, driving next-generation solutions for drug discovery and diagnostics.
Transform Your Studies with Cutting-Edge Fluorescent Products
| Catalog | Name | CAS | Inquiry |
|---|---|---|---|
| F01-0048 | 1,3,5,7,8-Pentamethyl-2,6-diethylpyrromethene-BODIPY | 131083-16-4 | Bulk Inquiry |
| F01-0054 | 8(4'-boronophenyl)-1,3,5,7-tetramethyl-BODIPY | 1254550-72-5 | Bulk Inquiry |
| F01-0056 | 1,3,5,7,8-Pentamethyl-2,6-dibormo-BODIPY | 134382-52-8 | Bulk Inquiry |
| F01-0057 | 4,4-Difluoro-8-bithienyl-1,3,5,7-tetramethyl-BODIPY | 1452398-18-3 | Bulk Inquiry |
| F01-0119 | 1,3,5,7-tetramethyl-2,6-diiodo-C3-SE-BODIPYs | 1825368-13-5 | Bulk Inquiry |
| F01-0131 | 3,5-di(4-hydroxylphenyl)vinyl-BODIPYs | 1421836-97-6 | Bulk Inquiry |
| F01-0148 | 3,5-divinylpyridyl-2,6-diiodo-BODIPYs | 1397771-30-0 | Bulk Inquiry |
| F01-0260 | BODIPY FL Phenyl Alkyne | 628729-80-6 | Bulk Inquiry |
| F01-0254 | BODIPY 493/503 carboxylic acid | 216961-95-4 | Bulk Inquiry |
| F01-0033 | 3-Bodipy-propanoic Acid Ethyl Ester | 1418610-53-3 | Bulk Inquiry |
High-Performance Fluorescent Tools for Your Research
- Alexa Fluor Bright, stable dyes for sensitive biosensing applications.
- Cyanine Versatile dyes used in biosensing and nucleic acid detection.
- Rhodamine Strong fluorescence, commonly used in protein and DNA sensing.
- Fluorescent Protein Used for live-cell imaging and real-time biosensing.
More About BODIPY Dyes
Online Inquiry

