Understanding and Controlling Fluorescence Quenching in BODIPY Dyes for Optimal Probe Design
BODIPY dyes, with their excellent photostability, high fluorescence quantum yields, and tunable spectral properties, have become indispensable fluorescent probes in bioimaging, optical sensing, and phototherapy research. However, in practical applications, fluorescence quenching is a critical factor that can affect BODIPY performance. Quenching not only reduces signal intensity but may also compromise probe selectivity and stability. Therefore, understanding quenching mechanisms, modulating structural factors, and implementing rational strategies are essential for developing high-performance BODIPY probes.
What is Fluorescence Quenching and Why Does It Matter?
In fluorescence chemistry and bioimaging research, the stability and intensity of the fluorescence signal are core indicators of probe performance. Even dyes with excellent optical properties, such as BODIPY, can be affected by fluorescence quenching. Quenching can arise from intrinsic molecular structural features or external environmental factors, such as solvent polarity, molecular concentration, or interactions with neighboring molecules. For researchers, quenching not only affects the fluorescence signal intensity but can also alter excited-state dynamics and probe response behavior, directly influencing the reliability of imaging, sensing, and therapeutic applications. Understanding the fundamental concepts and mechanisms of fluorescence quenching is a key starting point for optimizing BODIPY dye design, improving experimental success rates, and developing high-performance fluorescent probes.
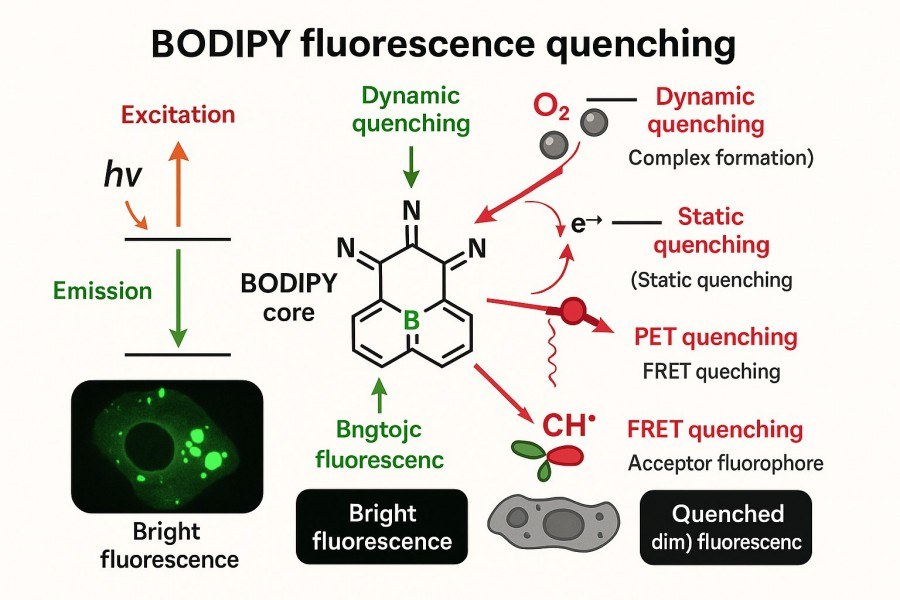 Fig. 1. BODIPY fluorescence quenching (BOC Sciences Authorized).
Fig. 1. BODIPY fluorescence quenching (BOC Sciences Authorized).
Definition and Mechanisms of Fluorescence Quenching
Fluorescence quenching refers to the reduction or complete loss of fluorescence intensity when a fluorescent molecule returns from the excited state to the ground state due to intermolecular interactions, environmental factors, or chemical reactions. Quenching affects not only the intensity of the optical signal but also the excited-state lifetime, thereby impacting the probe's sensitivity and detectability. In BODIPY dyes, quenching typically involves non-radiative decay pathways of excited-state electrons, including but not limited to:
- Collisional quenching: Excited BODIPY molecules collide with quenchers, releasing energy non-radiatively.
- Energy transfer: Excited-state energy is transferred to nearby molecules or acceptors, reducing fluorescence.
- Photoinduced electron transfer (PET): Electron transfer within or between molecules triggers non-radiative decay.
- Complex formation: Fluorescent molecules form stable non-fluorescent complexes with quenchers, preventing excited-state energy from being emitted as fluorescence.
Understanding these quenching mechanisms is fundamental to BODIPY probe design, helping scientists predict dye behavior under different solvents, concentrations, and environmental conditions, and ultimately optimize probe sensitivity and stability.
Static vs. Dynamic Quenching
Fluorescence quenching can be classified into static quenching and dynamic quenching.
- Static quenching: Occurs when a fluorescent molecule forms a non-fluorescent complex with a quencher in the ground state. The complex cannot emit fluorescence in the excited state. This type of quenching is usually related to molecular concentration and complex stability. In fluorescence lifetime measurements, it generally does not significantly alter excited-state lifetimes but decreases fluorescence intensity.
- Dynamic quenching: Also called collisional quenching, it occurs when excited-state molecules collide with quenchers, dissipating energy non-radiatively. Dynamic quenching significantly affects the excited-state lifetime, which shortens as quencher concentration increases.
For BODIPY dyes, distinguishing between static and dynamic quenching is critical. Different quenching mechanisms require distinct molecular design strategies: static quenching can be reduced by introducing steric hindrance or minimizing complex formation, while dynamic quenching can be controlled by adjusting excited-state electron distribution or enhancing molecular rigidity, thereby improving fluorescence efficiency and signal stability.
Importance in Imaging, Sensing, and Therapy Applications
Fluorescence quenching directly affects the performance of BODIPY dyes in bioimaging, molecular sensing, and photodynamic therapy (PDT).
- Bioimaging: Quenching leads to weakened fluorescence signals, reducing imaging contrast and resolution, particularly at high dye concentrations or in complex cellular environments.
- Molecular sensing: Quenching affects signal response sensitivity, potentially resulting in lower or unstable analytical readings. Controlling quenching behavior can enhance probe selectivity and detection accuracy.
- Photodynamic Therapy (PDT): Quenching directly impacts the efficiency of excited-state energy utilization, reducing the generation of singlet oxygen or radicals by photosensitizers and affecting therapeutic outcomes.
By systematically understanding and controlling fluorescence quenching mechanisms, researchers can optimize BODIPY dye performance to achieve bright, long-lived, and environmentally adaptable fluorescent probes, yielding more reliable results in both research and clinical studies. Additionally, quenching analysis provides guidance for structural modifications, such as introducing steric hindrance, tuning donor/acceptor strengths, or improving molecular solubility. Rational design strategies for quenching control not only enhance the practicality of BODIPY dyes but also accelerate experimental optimization and the development of new probes.
Quenching Pathways in BODIPY Dyes
In the application of BODIPY dyes, fluorescence quenching is a key factor influencing probe performance. To effectively design bright and stable fluorescent probes, researchers must understand the various pathways that may lead to quenching in BODIPY molecules. These pathways involve both intrinsic molecular structural features and interactions with the dye's environment. Mastering quenching pathways can guide molecular modifications, optimize fluorescence signals, and enhance the reliability of imaging, sensing, and phototherapy applications.
Aggregation-Induced Quenching (AIQ)
Aggregation-Induced Quenching (AIQ) is one of the most common quenching mechanisms in BODIPY dyes. When BODIPY molecules aggregate at high concentrations, in polar solvents, or in confined spaces, intermolecular π-π stacking causes excited-state energy to dissipate non-radiatively, significantly reducing fluorescence intensity. AIQ is particularly pronounced in water-soluble BODIPY dyes, as they more readily form aggregates in aqueous environments. Strategies to control AIQ include:
- Introducing bulky substituents on the molecular backbone to increase steric hindrance and reduce stacking potential;
- Adjusting the solvent or adding surfactants to improve molecular dispersion;
- Employing conjugation tuning or molecular modifications to optimize intermolecular interactions and maintain radiative decay of the excited state.
Photoinduced Electron Transfer (PET) Effects
Photoinduced Electron Transfer (PET) is another common quenching mechanism. When BODIPY molecules interact with nearby electron donors or acceptors, excited-state electrons may undergo non-radiative transfer, decreasing fluorescence emission. PET quenching is particularly important in functionalized BODIPY probes, as many sensor designs rely on donor/acceptor systems for signal response. Methods to regulate PET quenching include:
- Precisely tuning the electronic strength of donor or acceptor groups;
- Adjusting substituent positions to increase spatial separation;
- Optimizing molecular conjugation to distribute excited-state energy more evenly.
Energy Transfer to Acceptors (FRET, Dexter)
BODIPY dyes can also undergo quenching via energy transfer pathways, including FRET (Förster Resonance Energy Transfer) and Dexter energy transfer.
- FRET: Relies on spectral overlap and distance between donor and acceptor molecules; once excited-state energy is transferred to the acceptor, donor fluorescence is significantly reduced.
- Dexter energy transfer: A short-range transfer mechanism based on orbital overlap, typically requiring close molecular contact.
Energy transfer quenching is especially important in multi-dye systems and multifunctional probes, particularly in FRET sensors and multicolor imaging applications. By rationally designing intermolecular distances, spatial configurations, and energy-level alignment, non-radiative energy transfer can be effectively controlled to optimize fluorescence signal output.
Fluorescence Quenchers from BOC Sciences
| Solutions | Capabilities |
|---|---|
| Black Hole Quencher (BHQ) | Enables efficient, broad-spectrum fluorescence quenching with high stability for diverse molecular probes. |
| DusQ | Provides rapid, tunable quenching performance for optimized signal control in complex biological systems. |
| QSY | Offers reliable non-fluorescent energy dissipation across multiple wavelengths, supporting sensitive imaging and sensing applications. |
| Common Quenchers | Deliver versatile quenching solutions compatible with a wide range of fluorophores and experimental conditions. |
Structural Factors Influencing Quenching Behavior of BODIPY
The fluorescence performance of BODIPY dyes is influenced not only by external environments and quenching pathways but also by the intrinsic characteristics of their molecular structures. Understanding how structural factors affect quenching is essential for designing bright and highly stable probes. By rationally modulating the molecular backbone, substituents, and electronic effects, researchers can effectively reduce non-radiative energy dissipation and enhance BODIPY probe performance in complex biological systems.
Substituents at Meso, Alpha, and Beta Positions
The quenching behavior of BODIPY molecules is closely related to substituents at different positions on the molecular backbone. At the Meso position, introducing bulky substituents can significantly reduce intermolecular stacking, thereby decreasing aggregation-induced quenching (AIQ). Additionally, the electronic properties of Meso substituents can modulate excited-state electron density, influencing photoinduced electron transfer (PET) quenching. Substituents at the Alpha and Beta positions mainly affect the π-conjugation system and molecular planarity. By introducing appropriate substituents at these positions, the excited-state energy distribution can be optimized, reducing energy-transfer quenching while maintaining high quantum yields. Careful selection of substituent type and position is a key strategy for controlling quenching and enhancing fluorescence.
Influence of Conjugation and Planarity
The conjugation length and planarity of BODIPY molecules directly affect excited-state electron distribution and the probability of quenching. Extending the conjugation chain can enhance light absorption and emission efficiency, but excessive conjugation may increase the risk of intermolecular π-π stacking, leading to aggregation-induced quenching. Molecular planarity is equally important; fully planar BODIPY backbones tend to aggregate, whereas moderate twisting or the introduction of non-coplanar substituents can effectively suppress stacking-induced quenching while preserving effective conjugation. Molecular design must balance enhanced emission efficiency with reduced aggregation-induced quenching.
Role of Electron-Donating and Withdrawing Groups
Electron-donating and withdrawing groups significantly influence BODIPY quenching behavior. Electron-donating groups can stabilize excited-state electrons, reduce non-radiative dissipation, and lower the probability of PET quenching. Electron-withdrawing groups at specific positions may induce PET or energy-transfer quenching, but when strategically placed, they can also be used to construct environment-sensitive probes for controlled signal responses. By optimizing the type, position, and number of donor and acceptor groups, researchers can precisely tune BODIPY dye fluorescence intensity, lifetime, and quenching sensitivity, providing a reliable foundation for molecular design in research applications.
Strategies to Prevent or Control BODIPY Quenching
In BODIPY dye design and application, controlling quenching is critical for enhancing fluorescence intensity and stability. With a scientific understanding of quenching mechanisms, researchers can use structural modifications and molecular engineering approaches to effectively suppress non-radiative energy dissipation. This not only improves the optical performance of BODIPY probes but also increases their reliability in bioimaging, molecular sensing, and photodynamic therapy. The following are common strategies for controlling quenching and their molecular design principles.
Bulky Groups for Aggregation Inhibition
Introducing bulky substituents is a common approach to prevent aggregation-induced quenching (AIQ). Placing sterically large groups at the Meso or nearby positions can significantly reduce π-π stacking between BODIPY molecules, maintaining molecular dispersion in solution. This strategy is particularly important in high-concentration or aqueous environments, where BODIPY molecules are prone to forming non-fluorescent aggregates. By carefully selecting and positioning large substituents, researchers can maintain bright fluorescence signals without significantly affecting molecular optical properties.
Solubilizing Chains for Aqueous Stability
For water-soluble or biological applications, the dispersion and stability of BODIPY dyes are crucial for fluorescence performance. Introducing solubilizing chains, such as polyethylene glycol (PEG) or hydrophilic groups, can significantly improve dye stability in aqueous environments. These solubilizing chains help prevent molecular aggregation and reduce environment-induced quenching, maintaining stable fluorescence output. Furthermore, hydrophilic chains enhance the biocompatibility of dyes in cellular and in vivo environments, providing reliable support for imaging and sensing applications.
Tuning Excited-State Interactions via Molecular Engineering
Molecular engineering can control quenching by modulating excited-state interactions within or between molecules. For example, optimizing the conjugated system, adjusting substituent electronic properties, or introducing non-coplanar structures can effectively reduce non-radiative dissipation caused by PET or energy transfer. Additionally, precise control of intermolecular distances and spatial configurations helps minimize the risk of FRET or Dexter energy-transfer quenching. Through these strategies, researchers can fine-tune the excited-state dynamics of BODIPY, enabling the design of bright, long-lived, and environmentally adaptive fluorescent probes.
Experimental Techniques for Evaluating BODIPY Quenching
Accurately assessing fluorescence quenching is a critical step in developing and applying BODIPY dyes. By experimentally analyzing quenching mechanisms and intensity, researchers can evaluate the effectiveness of molecular designs and guide structural modifications and application optimization. Various techniques are used to assess quenching, including fluorescence lifetime measurements, quantum yield determination, Stern-Volmer analysis, and single-molecule or environment-sensitive quenching assays. These methods provide a comprehensive understanding of BODIPY probe excited-state behavior under different conditions.
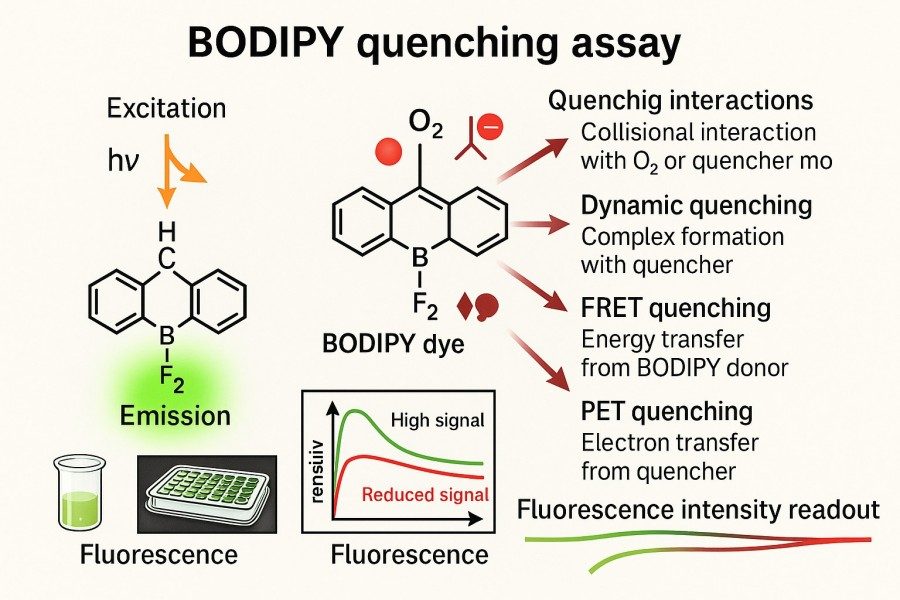 Fig. 2. BODIPY quenching assay (BOC Sciences Authorized).
Fig. 2. BODIPY quenching assay (BOC Sciences Authorized).
Fluorescence Lifetime and Quantum Yield Measurements
Fluorescence lifetime and quantum yield are fundamental parameters for evaluating quenching. Measuring excited-state lifetimes distinguishes dynamic from static quenching: dynamic quenching shortens the excited-state lifetime, while static quenching usually does not affect lifetime but reduces fluorescence intensity. Quantum yield measurements directly reflect the efficiency of converting absorbed light into fluorescence, serving as an important indicator of quenching. Combining lifetime and quantum yield data allows researchers to accurately quantify BODIPY dye quenching behavior under various solvents, concentrations, and environmental conditions, providing reliable guidance for molecular design and performance optimization.
Stern-Volmer Plots and Time-Resolved Spectroscopy
Stern-Volmer analysis is a classical method for quantifying fluorescence quenching. By plotting fluorescence intensity or lifetime against quencher concentration, the quenching type and efficiency can be determined. Time-resolved spectroscopy provides detailed information on excited-state dynamics, revealing intermolecular energy transfer, electron transfer, and non-radiative decay processes. Using these techniques, researchers can dissect BODIPY dye quenching mechanisms at the molecular level, providing quantitative support for quenching control strategies.
Single-Molecule and Environmental Quenching Assays
Single-molecule fluorescence analysis can reveal quenching phenomena that are difficult to capture in ensemble measurements, such as uneven molecular aggregation or local environmental variations. Environmental-sensitive quenching assays involve altering solvent polarity, pH, ionic strength, or adding acceptor molecules to observe BODIPY fluorescence responses, helping researchers understand molecular behavior in complex biological systems. These experimental approaches provide refined technical support for developing high-performance probes and optimizing imaging and sensing conditions.
Leverage Our Expertise in BODIPY Design and Optimization
BOC Sciences is dedicated to providing researchers and enterprises with high-performance BODIPY dyes and customized solutions. Whether for fundamental research or applied development, we offer molecular design optimization, performance tuning, and technical support tailored to your specific needs. By collaborating with us, you can access bright, low-quenching, and highly stable BODIPY dyes, along with comprehensive experimental and analytical guidance, accelerating the translation and application of your research findings.
Diverse Fluorescent Product Supply
- We offer an extensive library of BODIPY dyes, covering a wide range of excitation and emission wavelengths, functional modifications, and application scenarios.
- Customers can select the most suitable probes for their experiments without synthesizing from scratch, saving valuable R&D time.
- All products undergo rigorous quality control to ensure high purity and stability, providing reliable support for high-precision imaging and sensing.
Customized Dye Modification Services
- We provide tailored molecular modifications of BODIPY dyes, including steric group adjustments, electronic property optimization, and water-solubility enhancements.
- Our team of chemistry experts designs modification strategies based on your structural or performance requirements, maximizing fluorescence efficiency while controlling quenching effects.
- Support is available from small-scale research samples to pilot-scale production, meeting the needs of different stages of development.
Conjugation and Labeling Services
- BODIPY dyes can be efficiently conjugated to proteins, nucleic acids, lipids, or other biomolecules, ensuring stable and specific fluorescence signals.
- Optimized conjugation protocols and experimental guidance are provided to maintain high sensitivity and low background interference in imaging or sensing applications.
- These services are widely applied in fluorescent probe development, molecular tracking, and biosensing research.
Multifunctional Probe Development and Technical Support
- We support the development of multifunctional BODIPY probes, including FRET probes, environment-sensitive probes, and photodynamic applications.
- Comprehensive technical consultation is offered, including molecular design recommendations, quenching control strategies, and performance evaluation methods.
- Our one-stop solutions guide research teams from concept design to experimental validation, accelerating the translation of research findings and innovative applications.
Do You Need A Consultation?
BOC Sciences integrates cutting-edge fluorescence technologies to accelerate your research, driving next-generation solutions for drug discovery and diagnostics.
Transform Your Studies with Cutting-Edge Fluorescent Products
| Catalog | Name | CAS | Inquiry |
|---|---|---|---|
| F01-0166 | BODIPY 493/503 NHS Ester | 216961-98-7 | Bulk Inquiry |
| F01-0278 | BDY TR-X, SE | 197306-80-2 | Bulk Inquiry |
| F01-0036 | BDY 630-X, SE | 380367-48-6 | Bulk Inquiry |
| F01-0003 | BDP 558/568 carboxylic acid | 150173-72-1 | Bulk Inquiry |
| F01-0028 | BDY FL-X, SE | 217190-09-5 | Bulk Inquiry |
| F01-0220 | BODIPY TR-X NHS Ester | 217190-13-1 | Bulk Inquiry |
| F01-0156 | Pyrromethene 605 | 137829-80-2 | Bulk Inquiry |
| F01-0157 | Dipyrrometheneboron difluoride | 138026-71-8 | Bulk Inquiry |
| F01-0045 | BODIPY 505/515 | 21658-70-8 | Bulk Inquiry |
| F01-0234 | BDP FL-PEG4-amine | 2183473-14-3 | Bulk Inquiry |
High-Performance Fluorescent Tools for Your Research
- Alexa Fluor Bright, stable dyes for sensitive biosensing applications.
- Cyanine Versatile dyes used in biosensing and nucleic acid detection.
- Rhodamine Strong fluorescence, commonly used in protein and DNA sensing.
- Fluorescent Protein Used for live-cell imaging and real-time biosensing.
More About BODIPY Dyes
Online Inquiry

