Technical Guidance for Live and Fixed Cell Imaging with BODIPY Dyes
BODIPY fluorescent dyes, with their high brightness, excellent photostability, and outstanding cellular compatibility, hold an important position in modern cell imaging techniques. Whether for dynamic observation of live cell behavior or precise localization of molecular targets in fixed cells, BODIPY dyes demonstrate superior performance. To fully exploit their imaging potential, researchers need to scientifically optimize experimental design, dye selection, staining conditions, and imaging parameter settings. This article will systematically introduce the application techniques and key technical points of BODIPY fluorescent dyes in live and fixed cell imaging, helping researchers obtain high-quality and reproducible imaging results.
Understanding Live and Fixed Cell Imaging
In cell biology and microscopy research, understanding the basic principles and application significance of different imaging strategies is crucial for experimental design and dye selection. Live cell imaging and fixed cell imaging are currently the two main fluorescence imaging methods. Each has its own characteristics, challenges, and applicable scenarios. Reasonable combination of both can achieve a perfect integration of dynamic and static information.
Fig. 1. Live and fixed cell imaging (BOC Sciences Authorized).
What is Live Cell Imaging?
Live cell imaging refers to the imaging technique that uses fluorescent probes or labels to observe cellular internal structures, molecular dynamics, or signaling pathway activities in real-time while maintaining cell viability. This method can be used to:
- Observe processes such as cell migration, division, and apoptosis;
- Study cell signal transduction, protein localization, and transport;
- Analyze drug mechanisms of action and dose-response relationships;
- Monitor changes in the cellular microenvironment (such as pH, ROS, etc.).
Since it must be performed under non-destructive or minimally invasive conditions, live cell imaging imposes very high requirements on dyes: they must have good cell permeability, low phototoxicity, excellent photostability, and be capable of high signal-to-noise ratio imaging at relatively low concentrations.
What is Fixed Cell Imaging?
Fixed cell imaging is a technique where cells are "frozen" at a specific time point by chemical fixation methods (such as formaldehyde, alcohols, or crosslinkers), followed by fluorescence labeling and image acquisition. Its advantages include:
- Structural stability suitable for high-resolution imaging;
- Capability for multiple rounds of staining or restaining;
- Long-term sample preservation for repeated analysis;
- Superior spatial accuracy compared to live cell imaging.
Fixed imaging is widely applied in immunofluorescence staining, cell structure analysis, target colocalization studies, and more. However, fixation may affect cell morphology and antigen epitopes, so dye compatibility with fixation conditions is a critical consideration.
Differences and Complementary Use Cases
In practice, researchers often combine the two imaging methods. For example, live cell imaging can first track the movement of a protein, followed by fixed cell imaging to precisely locate its distribution within subcellular structures. Dynamic tracking before and after drug treatment can be performed with live cell imaging, then morphological and target verification analyses can be done by fixed cell imaging, thus obtaining more comprehensive research data.
Project Live Cell Imaging Fixed Cell Imaging Imaging Object Dynamic cells Static cells Imaging Time Real-time, continuous Single time point Fluorescent Dye Requirements Low toxicity, high photostability Structural stability, compatible with fixation conditions Applicable Research Cell behavior, dynamics analysis Structural observation, colocalization research Operational Complexity High, environment strictly controlled Relatively low, easier to handle
Common Challenges in Live and Fixed Cell Imaging
- Photobleaching and Phototoxicity Issues: Under high-energy illumination, fluorescent dyes easily undergo photobleaching, causing signal intensity decline and affecting imaging continuity. Meanwhile, some dyes or excitation lights may induce phototoxicity, triggering cellular stress responses or even cell death, severely compromising the reliability of experimental data.
- Maintaining Cell Viability During Imaging: In live cell imaging, any slight changes in solvents, dyes, or physical conditions may affect cell behavior. Therefore, dyes should have low toxicity, rapid staining, and no-wash features to minimize disturbance to the cellular microenvironment.
- Signal-to-Noise Ratio and Background Fluorescence: Background fluorescence mainly originates from cellular autofluorescence (e.g., NADH, flavins) and components in culture media. These signals interfere with specific probe emission, lowering imaging quality. Hence, dyes with high quantum yield and ideal excitation/emission wavelengths are required to improve the signal-to-noise ratio.
- Fixation Artifacts and Dye Compatibility: In fixed cell imaging, fixatives may cause cell morphology changes or antigen site loss, resulting in artifacts. Additionally, some dyes may degrade or deactivate during fixation, so probe development must consider compatibility with different fixation methods (e.g., formaldehyde, cold acetic acid).
Advantages of Using BODIPY Dyes for Cell Imaging
BODIPY dyes, short for boron-dipyrromethene, are a class of organic fluorescent dyes featuring a dipyrromethene backbone coordinated with a boron atom. Since the 1990s, due to their unique optical properties and highly tunable structures, BODIPY dyes have rapidly become one of the most important dyes in fluorescent probe development. They are widely used in cell imaging, biosensing, flow cytometry, drug screening, and photodynamic therapy in biomedical fields. Unlike many traditional dyes, BODIPY dyes exhibit excellent stability in both aqueous and organic phases, with fluorescence minimally affected by pH, polarity, or other microenvironmental factors. Therefore, BODIPY dyes are very suitable for constructing probes applicable to both live and fixed cells and can be conjugated with lipids, proteins, nucleic acids, carbohydrates, and other biological targets for high-sensitivity imaging.
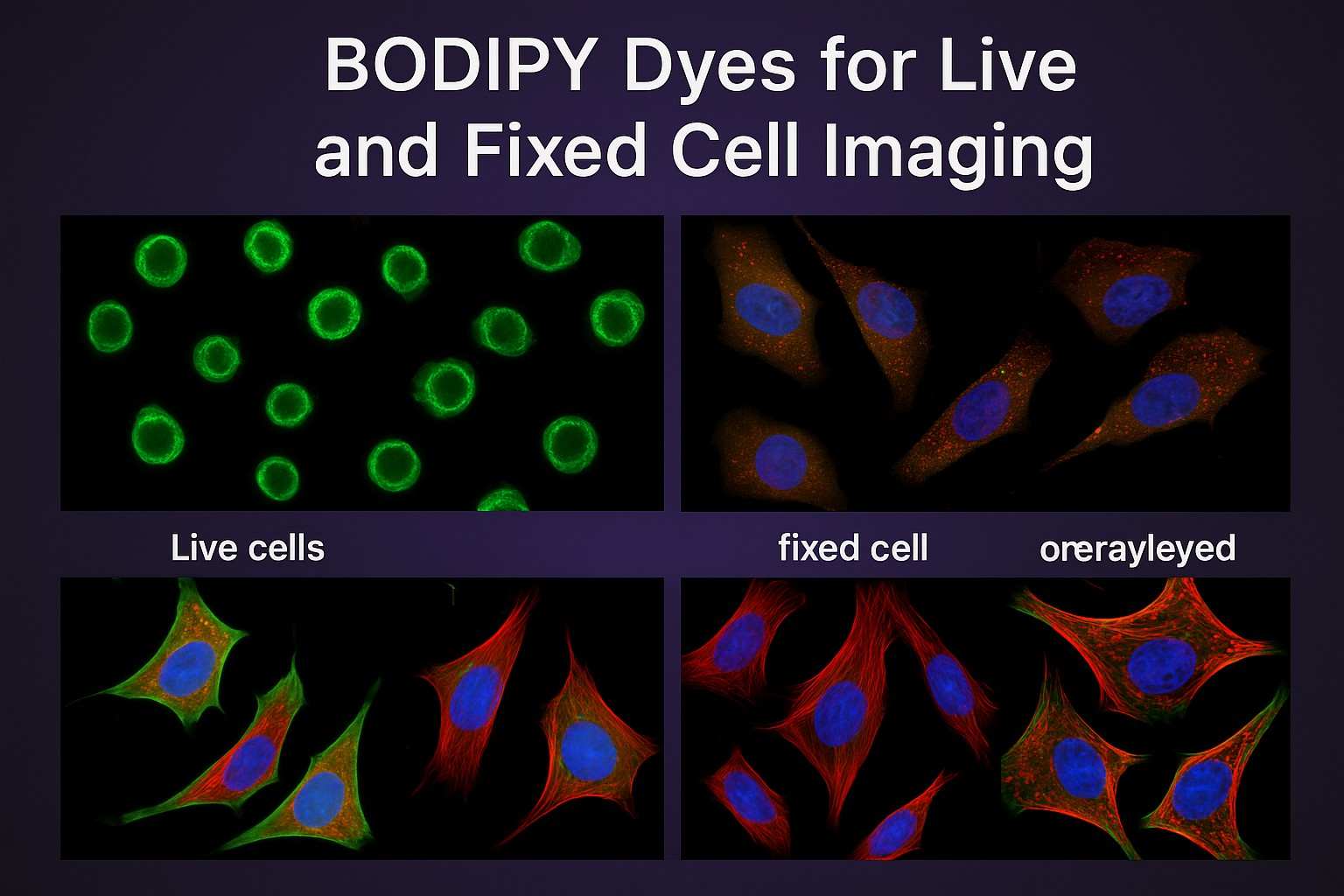
Fig. 2. BODIPY dyes in live and fixed cell imaging (BOC Sciences Authorized).
- Superior Photostability of BODIPY Fluorophores: The BODIPY structure features a rigid π-conjugated system that greatly enhances dye photostability, maintaining high emission efficiency even under strong or prolonged excitation. This characteristic makes it the ideal choice for long-term dynamic imaging.
- Bright Fluorescence and High Quantum Yield: BODIPY dyes have very high quantum yields (up to above 0.9), producing strong fluorescence signals upon excitation, significantly enhancing imaging sensitivity and resolution. Their narrow emission spectra and low background interference make them excellent for fine structural analysis and detecting weak signals.
- Versatility in Labeling Live and Fixed Cells: Through fine structural modification, BODIPY dyes' hydrophilicity, cell permeability, and conjugation ability with biomacromolecules can be controlled, enabling one-stop dye solutions usable in both live and fixed cells.
- Customizable Spectral Properties for Multiplexing: The emission wavelengths of BODIPY dyes can be precisely tuned from 500 to 700 nm through structural modifications, facilitating multiplex labeling with other fluorescent proteins or dyes. At the same time, their excitation spectra match well with conventional lasers, allowing seamless integration with existing imaging equipment.
BODIPY Dyes at BOC Sciences
| Cat. No. | Product Name | CAS No. | Inquiry |
|---|---|---|---|
| F01-0166 | BODIPY 493/503 NHS Ester | 216961-98-7 | Inquiry |
| F01-0211 | BODIPY FL Glibenclamide | N/A | Inquiry |
| F01-0165 | BODIPY FL, STP Ester, Sodium Salt | N/A | Inquiry |
| F01-0045 | BODIPY 505/515 | 21658-70-8 | Inquiry |
| F01-0172 | BODIPY FL L-Cystine | N/A | Inquiry |
| F01-0179 | BODIPY FL EDA | N/A | Inquiry |
| F01-0037 | azido-Bodipy-650 | N/A | Inquiry |
| F01-0038 | azido-Bodipy-FL-510 | 1048369-35-2 | Inquiry |
Practical Tips for Optimizing Live and Fixed Cell Imaging with BODIPY Dyes
BODIPY dyes are designed with high flexibility, but to achieve ideal imaging results in experiments, reasonable selection and optimization based on application scenarios are necessary. The following are several key practical strategies that help fully leverage the advantages of BODIPY probes in imaging, improving data quality and experimental reproducibility.
Selecting the Right Dye for Your Application
By customizing dye structures (such as adding hydrophilic groups or introducing targeting ligands), the compatibility of dyes with specific experimental needs can be further enhanced. When selecting BODIPY dyes, it is recommended to comprehensively consider the following factors:
- Target molecules or structures: Different modified types of BODIPY probes can be used for membrane imaging, mitochondrial imaging, nucleic acid detection, or lipid droplet imaging;
- Excitation/emission wavelength matching: Ensure the selected dye matches the laser channels and filters of the microscope system;
- Cell type and dye permeability: Some cell types uptake dyes slowly, so BODIPY variants with cell-penetrating modifications are recommended;
- Whether live cell imaging is required: For live cell imaging, dyes with low toxicity and no-wash protocols should be prioritized to avoid affecting cell viability;
- Multiplex labeling experimental needs: When multiple probes are used simultaneously, selecting BODIPY dyes with well-separated emission spectra is advisable.
Optimizing Staining Protocols for Live Cells
To ensure that dyes rapidly, uniformly, and with minimal interference label targets in live cells, the following strategies are recommended:
- Use serum-free culture medium for staining: To reduce nonspecific binding between serum proteins and dyes, lowering background;
- Control dye concentration and incubation time: BODIPY dyes provide strong signals at nanomolar levels, generally recommended concentrations are 50–500 nM with incubation times of 5–30 minutes;
- Avoid light exposure: Prevent strong light during staining to avoid premature photobleaching;
- Choose no-wash dyes: Some BODIPY probes can be imaged directly after staining, saving operation time and reducing cell damage;
- Low-temperature staining (optional): For metabolically active cells or experiments that require limiting endocytosis, staining at 4 °C can maintain dye localization accuracy.
Best Practices for Fixation and Staining of Fixed Cells
Successful fixed cell imaging depends on good preservation of cell structure and dye signal retention. The following points are recommended:
- Select appropriate fixatives: 4% paraformaldehyde is the most commonly used fixative suitable for most BODIPY dyes; for structure-sensitive applications, mild fixatives such as acetic acid-ethanol can be considered;
- Clear staining sequence: Some BODIPY dyes are suitable for "stain first, then fix" to maximize dye uptake efficiency; however, most scenarios still recommend "fix first, then stain" to control target stability;
- Blocking and permeabilization treatment: Adding 0.1–0.3% Triton X-100 helps dye permeate fixed cells; blocking steps reduce nonspecific binding;
- Thorough washing after staining: Washing with PBS or 0.1% Tween-20 helps reduce background signals and improve imaging clarity;
- Pay attention to dye fixation compatibility: Some BODIPY dyes may be sensitive to certain crosslinkers and require prior testing of fluorescence retention.
Imaging Settings to Maximize Fluorescence and Minimize Artifacts
Fluorescence microscopy parameter settings significantly affect final image quality. To maximize BODIPY dye fluorescence performance, it is recommended to:
- Match appropriate excitation sources and filter sets: Set correct channels according to dye excitation/emission wavelengths, avoiding excessive excitation energy or insufficient detection efficiency;
- Use low excitation power and short exposure time: Control exposure conditions to reduce photobleaching while maintaining signal strength;
- Enable real-time autofocus and exposure control: Prevent image deviations caused by focus changes or uneven excitation;
- Apply image post-processing algorithms: Such as deconvolution or background subtraction to significantly improve signal-to-noise ratio;
- Avoid spectral crosstalk: For multiplex imaging, use sequential scanning or spectral unmixing tools to reduce fluorescence overlap errors.
BODIPY Dye Services for Live and Fixed Cell Imaging
BOC Sciences is committed to providing high-quality molecular tools and customized solutions for life science research and drug development. In the R&D and application of BODIPY dyes and derived probes, we not only have a mature synthesis platform but also deep expertise in structural design and application optimization.
Custom BODIPY Probe Development Services
- Design various functional groups (such as carboxyl, amino, azido, alkyne, maleimide, etc.) according to customer needs to achieve covalent coupling or click chemistry labeling;
- Synthesize dyes emitting green, orange, red, or near-infrared through molecular structure adjustment to meet multi-channel confocal requirements;
- Conjugate BODIPY dyes with antibodies, peptides, oligonucleotides, carbohydrates, or lipids to achieve specific localization;
- Develop responsive probes sensitive to pH, redox state, ion concentrations (e.g., Ca²⁺, Zn²⁺) for dynamic cell function studies.
Technical Support for Dye Selection and Protocol Optimization
- Recommend the most suitable BODIPY dye series based on customer cell type, target distribution, and experimental goals;
- Provide detailed staining steps, dye concentration suggestions, buffer system configuration for live or fixed cell imaging;
- Offer reasonable dye combinations and excitation/emission channel suggestions for multiplex experiments to avoid signal overlap;
- Help analyze causes of staining failure or unstable signals and propose improvement strategies (such as changing fixatives or improving permeabilization).
Compatibility Testing with Cell Types and Imaging Platforms
- Evaluate dye signal performance and impact on cell viability in HeLa, 293T, CHO, RAW264.7, U2OS cells, etc.;
- Verify dye stability after treatment with various fixatives (e.g., PFA, methanol);
- Includes compatibility testing with laser confocal microscopes (Zeiss, Leica, Nikon, Olympus), widefield fluorescence microscopes, and high-content imaging systems;
- Assess dye matching with platform excitation wavelengths and detection filters, providing signal-to-noise ratio and imaging quality reference data.
Do You Need A Consultation?
BOC Sciences integrates cutting-edge fluorescence technologies to accelerate your research, driving next-generation solutions for drug discovery and diagnostics.
Versatile Fluorophores for Modern Labs
| Cat. No. | Product Name | CAS No. | Inquiry |
|---|---|---|---|
| F01-0053 | 8(4'-bromophenyl)-1,3,5,7-tetramethyl-BODIPY | 850534-66-6 | Inquiry |
| F01-0212 | Vancomycin, BODIPY FL Conjugate | N/A | Inquiry |
| F01-0048 | 1,3,5,7,8-Pentamethyl-2,6-diethylpyrromethene-BODIPY | 131083-16-4 | Inquiry |
| F01-0169 | BODIPY FL N-(2-Aminoethyl)Maleimide | N/A | Inquiry |
| F01-0199 | Cytochalasin D, BODIPY TMR Conjugate | N/A | Inquiry |
| F01-0054 | 8(4'-boronophenyl)-1,3,5,7-tetramethyl-BODIPY | 1254550-72-5 | Inquiry |
| F01-0056 | 1,3,5,7,8-Pentamethyl-2,6-dibormo-BODIPY | 134382-52-8 | Inquiry |
| F01-0057 | 4,4-Difluoro-8-bithienyl-1,3,5,7-tetramethyl-BODIPY | 1452398-18-3 | Inquiry |
| F01-0099 | 3,5-divinylthienyl-BODIPYs | N/A | Inquiry |
| F01-0119 | 1,3,5,7-tetramethyl-2,6-diiodo-C3-SE-BODIPYs | 1825368-13-5 | Inquiry |
Next-Level Fluorescent Tools for Modern Science
- Fluorescence Imaging Versatile dyes for high-contrast, multicolor fluorescence detection.
- Immunofluorescence Staining Antibody-compatible dyes for specific target visualization in cells and tissues.
- Cell Staining Comprehensive fluorescent stains for membranes, nuclei, and organelles.
- Fluorescence Microscopy High-performance probes designed for bright, photostable microscopic imaging.
More About BODIPY Dyes
Online Inquiry

