Rhodamine Spectral Properties and Fluorescence Mechanism Explained for Research Use
Rhodamine dyes, as core fluorophores in bioanalytical and optical imaging applications, have become one of the most widely used fluorescent probe families in chemical biology research. This is due to their excellent molar extinction coefficients, extremely high quantum yields, and outstanding photophysical stability in complex physiological environments. These dyes are based on the rigid xanthene core structure, which effectively suppresses non-radiative energy dissipation, enabling high-brightness photon emission in the excited state. A deep understanding of rhodamine's spectral properties and the underlying electronic transition mechanisms is critically important for researchers to precisely control experimental parameters, develop novel fluorescent sensors, and enhance the sensitivity of biomolecular detection.
Introduction to Rhodamine Dyes for Research Applications
In modern biochemical and biophysical research, fluorescent probes are essential tools for achieving high-sensitivity detection and visualizing microscopic structures. Among many organic fluorophores, the rhodamine family stands out in life science research due to its exceptional quantum efficiency, physicochemical stability, and highly tunable structure. From its early use as an industrial tracer to its current role as a key probe in super-resolution microscopy, the evolution of rhodamine reflects the ongoing pursuit of high-brightness and high-contrast imaging in bioanalytical techniques.
Why Rhodamine Is Popular in Fluorescence Research?
Rhodamine maintains a central role in demanding research applications thanks to its unique xanthene heterocyclic core. This highly planar and rigid tricyclic system not only provides very high molar extinction coefficients (typically ɛ > 80,000 M⁻¹cm⁻¹) but also ensures that excited-state electrons efficiently convert energy into photon emission.
- Outstanding photophysical properties: Compared with fluorescein, which is sensitive to environmental pH fluctuations, most rhodamine derivatives exhibit highly consistent spectra within physiological pH ranges.
- High-brightness output: Rhodamine provides exceptionally strong signals in the visible light range. Its strong absorption between 540 nm and 600 nm allows optimal matching with common laser sources, such as 532 nm or 561 nm.
- Structural tunability: By introducing different amino or heterocyclic substituents at the 3 and 6 positions of the xanthene ring, researchers can fine-tune the electron density, shifting the emission wavelength from yellow-green to the near-infrared region, meeting multi-color labeling requirements.
How Rhodamine Helps Overcome Signal Loss and Photobleaching?
Photobleaching is a major factor limiting data reliability in fluorescence experiments, especially during long-term dynamic tracking studies. Rhodamine probes enhance photostability through multiple chemical strategies:
- Molecular rigidity: Chemically locking the side-chain amines of rhodamine into saturated ring structures (e.g., replacing diethylamino groups with nitrogen heterocycles) suppresses vibrational relaxation (internal conversion). This structural locking reduces energy dissipation as heat, increasing quantum yield and extending the molecule's photon budget.
- Electronic shielding effect: Modern modified rhodamines, such as fluorinated rhodamines, introduce electron-withdrawing groups on the molecular backbone, slowing oxidative reactions with reactive oxygen species (ROS) and preventing fluorescence quenching.
- Resistance to quenching: Rhodamine often maintains a longer fluorescence lifetime when bound to biomacromolecules (e.g., proteins or antibodies), offering a significant advantage for background-suppressed imaging in complex cellular environments.
Selecting the Right Rhodamine Dye for Your Experiment
When faced with numerous rhodamine derivatives, researchers must balance spectral overlap, cell permeability, and photostability based on experimental goals. Selecting the appropriate fluorescent dye is key to experimental success. Factors such as the experimental objective, sample type, imaging equipment, and detection channels should all be considered. The rhodamine family includes a variety of derivatives, each differing in excitation/emission wavelengths, hydrophilicity, cell permeability, and conjugation options. Properly matching the derivative to experimental needs maximizes fluorescent signal, improves the signal-to-noise ratio, and minimizes background interference. Below is a list of commonly used rhodamine derivatives and typical application examples for reference.
| Rhodamine Dye | Excitation (nm) | Emission (nm) | Water Solubility | Common Applications | Notes |
|---|---|---|---|---|---|
| TRITC (Tetramethylrhodamine Isothiocyanate) | 550 | 570 | Moderate | Antibody labeling, immunofluorescence, confocal microscopy. | Isothiocyanate group allows covalent binding to amines on proteins. |
| Rhodamine B | 540 | 625 | Low | Cell staining, flow cytometry, general fluorescence imaging. | Lipophilic, often used for membrane labeling. |
| Rhodamine 6G | 530 | 555 | Moderate | Live-cell imaging, mitochondrial labeling, FRET studies. | High quantum yield, excellent photostability. |
| Rhodamine 110 | 496 | 520 | High | Nucleic acid labeling, enzyme assays. | Water-soluble, suitable for aqueous biological samples. |
| Rhodamine 123 | 507 | 529 | Moderate | Mitochondrial staining, live-cell imaging, flow cytometry. | Specifically accumulates in mitochondria, useful for membrane potential studies. |
| Rhodamine 101 | 586 | 605 | Low | High-sensitivity fluorescence imaging, flow cytometry. | Bright and photostable, suitable for single-molecule and quantitative imaging. |
| Tetramethylrhodamine (TMR) | 555 | 580 | Moderate | Protein labeling, fluorescence microscopy. | Compatible with FRET experiments. |
Looking for Rhodamine Dyes?
Whether you require specialized Rhodamine derivatives, water-soluble variants, or photostable forms, our experts offer tailored synthesis solutions to meet your research and experimental needs.
Key Spectral Properties of Rhodamine: Excitation and Emission
Spectral properties are the fundamental parameters defining the performance of a fluorescent dye. In biochemical research, understanding the absorption and emission dynamics of rhodamine not only helps optimize detection system hardware (such as filter and laser matching) but also forms the basis for precise quantitative fluorescence analysis. The spectral characteristics of rhodamine dyes are directly governed by their conjugated electronic system, exhibiting high molar extinction coefficients and excellent photon conversion efficiency.
Absorption and Excitation Wavelengths
The characteristic absorption bands of the rhodamine family are primarily in the green to yellow region (500–560 nm). Their electronic transitions are mainly π→π* type, attributed to the delocalized π-electron system on the xanthene ring.
- Excitation efficiency: For example, the classic Rhodamine 6G has a maximum absorption wavelength (λabs) around 530 nm, perfectly matching common 532 nm solid-state lasers, achieving near-peak excitation efficiency.
- Molar extinction coefficient (ɛ): Rhodamine's ɛ typically ranges from 80,000 to 120,000 M⁻¹cm⁻¹, meaning that even at very low concentrations, the molecule can capture a large number of photons, significantly enhancing detection sensitivity—a critical factor for tracking low-abundance proteins.
Emission Characteristics and Stokes Shift
Rhodamine's emission spectra generally mirror the absorption spectra, with emission peaks (λem) located in the red region.
- Spectral purity: Rhodamine exhibits narrow emission bandwidths (FWHM typically 30–50 nm), which reduces spectral bleed-through between adjacent channels in multicolor fluorescence imaging experiments.
- Stokes shift: Rhodamine shows relatively small Stokes shifts, usually ranging from 20 to 40 nm. Although small shifts require steeper filter edges, the narrow absorption peak allows high-quality dichroic mirrors to effectively separate excitation light from fluorescence signals.
Quantum Yield and Photostability
Quantum yield (Φ) measures a fluorescent molecule's ability to convert absorbed light into fluorescence, a key indicator of dye “brightness.”
- Near-perfect conversion: In solvents such as ethanol, Rhodamine 6G can reach a quantum yield of up to 0.95, meaning almost every absorbed photon is converted into fluorescence emission.
- Photostability: The rigid aromatic structure of rhodamine enhances both emission efficiency and resistance to light-induced degradation. Compared with organic small molecules that rapidly fade under singlet oxygen attack, rhodamine maintains effective signal for longer under continuous high-power laser scanning.
- Environmental dependence: Although relatively stable, rhodamine's spectral properties can be influenced by solvent polarity, ionic strength, and temperature. For example, in low-polarity environments, some rhodamine derivatives may experience spectral blue shifts or increased quantum yield.
Fluorescence Mechanism of Rhodamine Explained
Rhodamine fluorescence fundamentally results from the competition between electronic transitions and energy dissipation within the molecule. By studying the relationship between molecular conformation and electron distribution, researchers can better understand how environmental factors (e.g., polarity, viscosity, pH) modulate fluorescence output, enabling more accurate measurements in complex biological samples.
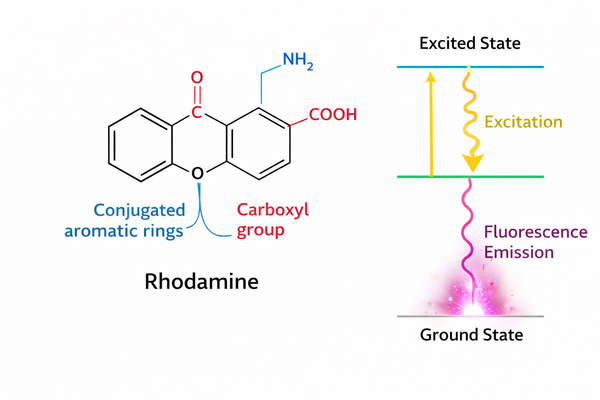 Fig. 1. Schematic of rhodamine molecular structure and electron transitions (BOC Sciences Authorized).
Fig. 1. Schematic of rhodamine molecular structure and electron transitions (BOC Sciences Authorized).
Molecular Structure and Electron Transitions
The core of rhodamine consists of a central xanthene ring connected to an ortho-substituted benzene at the C9 bridge position. Its high emission efficiency derives from the extensively delocalized π-electron system.
- Energy levels: In the ground state (S0), electrons occupy the highest occupied molecular orbital (HOMO). Upon energy absorption, electrons transition to the lowest unoccupied molecular orbital (LUMO), the excited state S1.
- Push-pull system: The 3 and 6 positions of the rhodamine scaffold typically carry amino or substituted amino groups. These electron-donating groups form a charge-delocalized system with the electron-deficient xanthene core. Upon excitation, electron density redistributes across the xanthene ring, enhancing the transition dipole strength and defining the molecule's high absorption cross-section.
- Structural rigidity and non-radiative transitions: Fluorescence efficiency depends on the competition between radiative (emission) and non-radiative (thermal dissipation) processes. The planar rigidity of the xanthene core restricts internal rotations and vibrations, forcing excited-state energy to release as photons, explaining rhodamine's near-unity quantum yield.
Excitation and Emission Process
Rhodamine's optical behavior follows the Jablonski kinetic model, cycling from ground to excited states and back.
- Absorption and excitation: When photons of matching wavelength enter, electrons move from the lowest vibrational level of S0 to a higher vibrational level of S1 within ~10⁻¹⁵ s, following the Franck-Condon principle.
- Vibrational relaxation and internal conversion: Electrons at high S1 levels relax to the lowest vibrational level via solvent collisions in ~10⁻¹² s, causing the fluorescence wavelength to be longer than the excitation light (Stokes shift).
- Fluorescence emission: Electrons return from the bottom of S1 to S0, releasing photons. With S1 lifetimes of a few nanoseconds, rhodamine is ideal for time-resolved fluorescence imaging (FLIM).
Factors Affecting Fluorescence Efficiency
Despite its stability, several physicochemical factors affect rhodamine's fluorescence efficiency, resulting in “environmental sensitivity.”
- Spirolactonization: In acidic or polar environments, rhodamine remains in an open conjugated state with strong fluorescence. In alkaline or nonpolar conditions, the C9 carboxyl may attack to form a closed spirolactone, disrupting conjugation and rendering the molecule non-fluorescent.
- Concentration quenching and dimerization: At concentrations above a threshold (usually micromolar), planar molecules stack via π–π interactions, forming H-dimers that deactivate primarily through non-radiative pathways, causing fluorescence quenching.
- Heavy atom effects and quenchers: Heavy metal ions (e.g., Hg²⁺, Cu²⁺) or dissolved oxygen enhance intersystem crossing (ISC) from S1 to T1, reducing fluorescence intensity and generating long-lived reactive oxygen species, which accelerate photobleaching and may affect cells.
- Solvent viscosity and temperature: In low-viscosity or high-temperature environments, internal rotations (e.g., of the C9 phenyl ring) increase, opening non-radiative decay pathways and lowering quantum yield.
Applications of Rhodamine in Biomedical and Life Science Research
Rhodamine dyes are widely used in biomedical, molecular biology, and cellular imaging research due to their high brightness, photostability, and facile conjugation. By precisely labeling proteins, nucleic acids, or cellular structures, researchers can achieve high-sensitivity detection, quantitative analysis, and multiplexed experimental designs.
Protein and Nucleic Acid Labeling
Rhodamine dyes can be efficiently conjugated to proteins, antibodies, or nucleic acids to create stable labels. In protein detection assays such as Western Blot, ELISA, and immunofluorescence, rhodamine-labeled antibodies provide high signal-to-noise detection, significantly enhancing experimental sensitivity. In nucleic acid research, rhodamine probes enable precise labeling of DNA or RNA fragments, facilitating fluorescence detection in techniques such as FISH and real-time PCR. Moreover, the diverse derivatives and conjugation options allow scientists to select the most suitable dye type for their experimental needs, ensuring labeling efficiency and functional integrity.
Cellular Imaging and Live-Cell Fluorescence
In cellular imaging, rhodamine derivatives (e.g., Rhodamine 123, TRITC) can label mitochondria, endoplasmic reticulum, lysosomes, and other subcellular structures for high-resolution imaging. Their low cytotoxicity and excellent photostability allow long-term dynamic observation in live-cell experiments, such as monitoring mitochondrial membrane potential changes or organelle movement. Rhodamine dyes are compatible with confocal microscopy, fluorescence microscopy, and super-resolution imaging techniques, providing nanometer-scale spatial resolution and enabling researchers to study subcellular structures and dynamic physiological processes.
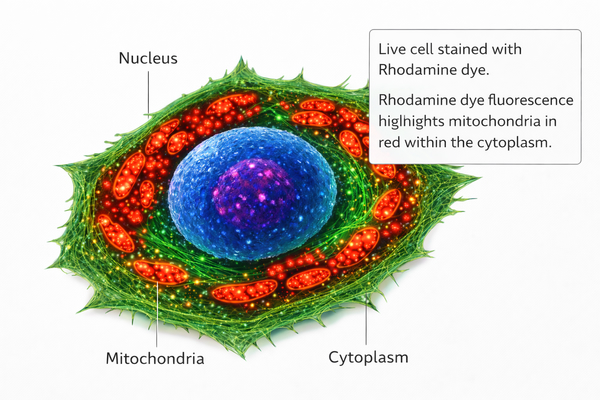 Fig. 2. Schematic representation of rhodamine dye distribution in live-cell imaging (BOC Sciences Authorized).
Fig. 2. Schematic representation of rhodamine dye distribution in live-cell imaging (BOC Sciences Authorized).
Multiplexed Fluorescence Assays
Rhodamine dyes exhibit excellent compatibility in multiplexed fluorescence experiments. Their specific excitation and emission bands allow simultaneous use with other fluorophores (e.g., FITC, Cy5) for multi-target detection within a single sample. For example, in immunohistochemistry or flow cytometry, multiple protein markers can be detected concurrently, significantly increasing experimental throughput and information content. Properly designed multiplexed assays benefit from rhodamine's Stokes shift and photostability, minimizing signal crosstalk and improving the accuracy and reliability of quantitative analysis.
Fluorescence Resonance Energy Transfer (FRET) Studies
In FRET experiments, rhodamine is commonly used as an energy donor or acceptor, with its high quantum yield and stability ensuring efficient energy transfer. Researchers can use rhodamine-based FRET systems to study protein-protein interactions, molecular conformational changes, and signaling pathways. For example, in drug screening or signal pathway analysis, rhodamine-labeled molecules can accurately reflect dynamic interactions, providing quantitative data for mechanistic studies.
Flow Cytometry and Cell Sorting
Rhodamine dyes can be used in flow cytometry to label cell surface receptors or intracellular targets, enabling high-throughput analysis and precise cell sorting. Their high brightness and photostability maintain clear signals even in complex samples or multi-parameter analyses. For example, in immunology research, rhodamine-labeled antibodies can distinguish different immune cell subpopulations and, when combined with functional assays, allow quantitative evaluation of cellular responses.
Fluorescence Microscopy of Subcellular Structures
Rhodamine can label subcellular structures such as the plasma membrane, mitochondria, endoplasmic reticulum, and Golgi apparatus for detailed imaging with fluorescence microscopy. When combined with confocal or super-resolution microscopy, rhodamine dyes provide high-contrast, high-resolution images, helping researchers analyze the spatial distribution and dynamic behavior of intracellular structures. Their photostability ensures that signals remain consistent during long-term imaging, facilitating continuous tracking of cellular processes.
Optimizing Rhodamine Fluorescence in Experiments
Although rhodamine performs exceptionally well, its fluorescence is strongly influenced by environmental factors and experimental conditions. To obtain reproducible, high-quality quantitative data, researchers must optimize dye selection and experimental workflows based on specific needs, balancing signal intensity with sample integrity.
Selecting the Right Rhodamine Derivative
Not all rhodamine derivatives are universally applicable in complex experimental designs. For a given biological system, careful selection based on physicochemical properties is essential:
- Solubility considerations: In vivo applications or high-concentration stock solutions require dyes with adequate solubility, which directly impacts biodistribution and labeling efficiency. Traditional rhodamines are often hydrophobic and prone to aggregation in aqueous buffers. Polar derivatives, such as sulfonated dyes (e.g., Sulforhodamine 101/Texas Red), improve water solubility and reduce non-specific adsorption to cell membranes or plasticware, lowering background noise.
- Wavelength matching and tissue penetration: Optical hardware sets limits on signal collection. Researchers should match dyes to available laser lines (e.g., 532 nm or 561 nm) and filter cubes. While TMR is highly stable, longer-wavelength rhodamine derivatives like Texas Red perform better in thick tissue samples, as their red-shifted emission reduces scattering and absorption, enabling deeper imaging.
- pH stability and microenvironment sensitivity: Experiments involving acidic compartments, such as lysosomes (pH 4.5–5.0) or endosomes, require pre-assessment of dye photophysical stability. Some rhodamine derivatives protonate under acidic conditions, enhancing fluorescence or shifting emission, while others may lose activity due to spirolactone formation. Ensuring a stable quantum yield within the target pH range is crucial for accurate quantitative analysis.
Avoiding Photobleaching and Signal Loss
To maximize the photon budget and capture high signal-to-noise images during limited experimental windows, the following strategies help combat photobleaching:
- Adding antifade reagents: Photobleaching is often caused by excited-state dye interactions with oxygen, generating singlet oxygen. Including reagents such as n-propyl gallate (PPG), DABCO, or commercial antifade buffers in mounting media or live-cell imaging buffers effectively scavenges reactive oxygen species, extending fluorophore lifetime for long-term dynamic observation.
- Optimizing laser excitation intensity: Leveraging rhodamine's high molar extinction coefficient and sensitivity, initial laser power should be minimized (typically 1–5%) to reduce phototoxicity. Exposure time or detector gain can then be adjusted to achieve satisfactory images without damaging live cells.
- Controlling the F/P ratio (dye/protein conjugation ratio): In immunofluorescence probes, higher labeling is not always better. Excessive labeling (F/P > 4) can induce self-quenching, dissipating energy non-radiatively between adjacent rhodamine molecules. Careful optimization of dialysis and purification maintains an ideal F/P ratio, ensuring maximal brightness per labeled protein.
Buffer and Environmental Considerations
The chemical environment subtly but decisively affects fluorescence efficiency:
- Ionic interference and quenching effects: Rhodamine fluorescence is sensitive to certain metal ions (e.g., Fe³⁺, Cu²⁺, Hg²⁺), which increase non-radiative dissipation via intersystem crossing. Using ultrafiltered, conductivity-controlled physiological buffers is recommended for precise quantitative experiments. Chelators such as EDTA may be added when metal ions are unavoidable.
- Autofluorescence suppression and spectral separation: Thick tissue or plant samples often exhibit strong autofluorescence in the 400–500 nm range due to collagen, riboflavin, and flavoproteins. Using red-shifted rhodamine probes (e.g., Si-Rhodamine, with near-infrared excitation/emission) avoids the autofluorescence window. Narrow-band interference filters can then completely separate target signals from background, achieving high spatial resolution.
Custom Rhodamine Support Services at BOC Sciences
BOC Sciences has established a comprehensive rhodamine R&D, production, and technical support system. We not only provide high-purity catalog reagents but also leverage extensive organic synthesis expertise and bioconjugation experience to solve core technical challenges in fluorescence detection, making us a strategic partner in fluorescent probe development.
Comprehensive Rhodamine Product Selection
- Classic rhodamine series: High-purity Rhodamine 6G, Rhodamine B, and Tetramethylrhodamine (TMR/TAMRA), suitable for standard immunofluorescence and routine biochemical assays.
- Sulfonated derivatives: Sulforhodamine 101 (Texas Red) offers excellent water solubility and minimal non-specific adsorption, ideal for thick tissue imaging.
- Advanced imaging probes: Si-Rhodamine (SiR) series optimized for STED super-resolution microscopy, and rhodamine spirolactone derivatives with photo-switchable properties.
Rhodamine Synthesis and Modification Service
- Spectral fine-tuning: By introducing fluorine atoms or altering nitrogen heterocycles on the xanthene ring, we can customize probes with specific excitation/emission wavelengths.
- Solubility and affinity improvement: PEG chains or sulfonate groups can be added to optimize probe diffusion and biocompatibility in physiological environments.
- Multifunctional modifications: Development of rhodamine precursors combining fluorescence imaging, targeted recognition, and photodynamic therapy capabilities.
Custom Conjugation and Labeling Solution
- Biomacromolecule labeling: Rhodamine-labeled dextrans, BSA, and recombinant proteins with precisely controlled F/P ratios.
- Small molecule and lipid conjugation: Rhodamine-labeled phospholipids, cholesterol, and synthetic polymers (e.g., PLGA, PEG) for drug delivery studies.
- Surface modification services: Functionalization of nanoparticles, magnetic beads, and microplate surfaces with rhodamine for high-throughput screening and biosensor development.
Technical Consultation and R&D Support
- Protocol optimization consulting: Guidance on dye selection and background suppression strategies to address photobleaching, spectral overlap, and autofluorescence issues.
- FRET system design: Assistance in calculating Förster distances and selecting optimal donor-acceptor pairs for high-sensitivity energy transfer experiments.
- Full-process quality assurance: All products come with detailed HPLC purity, mass spectrometry verification, and spectral analysis reports, ensuring internationally recognized rigor and reproducibility of your research data.
Do You Need A Consultation?
BOC Sciences integrates cutting-edge fluorescence technologies to accelerate your research, driving next-generation solutions for drug discovery and diagnostics.
Transform Your Studies with Cutting-Edge Fluorescent Products
| Catalog | Name | CAS | Inquiry |
|---|---|---|---|
| A17-0047 | Rhodamine 700 perchlorate | 63561-42-2 | Bulk Inquiry |
| A03-0012 | Dihydrorhodamine 123 | 109244-58-8 | Bulk Inquiry |
| A17-0016 | Rhodamine 6G Perchlorate | 13161-28-9 | Bulk Inquiry |
| A17-0062 | Rhodamine 3B Perchlorate | 23857-69-4 | Bulk Inquiry |
| A18-0008 | Rhodamine 110 chloride | 13558-31-1 | Bulk Inquiry |
| A14-0059 | N-hydroxy Rhodamine B amide | 1115867-62-3 | Bulk Inquiry |
| A14-0060 | Rhodamine B thiospirolactone | 111883-10-4 | Bulk Inquiry |
| A16-0142 | Dihydrorhodamine 6G | 217176-83-5 | Bulk Inquiry |
| A16-0149 | Rhodamine B hexyl ester perchlorate | 877933-92-1 | Bulk Inquiry |
| F05-0007 | Carboxyrhodamine 110-PEG4-alkyne | 2055103-66-5 | Bulk Inquiry |
High-Performance Fluorescent Tools for Your Research
- BODIPYBright, stable dyes used in bioimaging and molecular labeling.
- CoumarinBlue-emitting dyes for chemical sensing and fluorescence studies.
- CyanineVersatile dyes used in biosensing and nucleic acid detection.
- Fluorescent ProteinUsed for live-cell imaging and real-time biosensing.
More About Rhodamine Dyes
Online Inquiry

