Fluorescent-Labeled Antibodies for Protein Visualization and Localization Analysis
In modern protein research, scientists are no longer satisfied with the single question of whether a protein is expressed. Instead, greater attention is paid to its spatial distribution, expression patterns, dynamic changes, and interactions within cells or tissues. In this context, fluorescent-labeled antibodies have become a central tool linking molecular recognition to imaging analysis. By covalently conjugating specific fluorescent dyes to highly specific antibodies, researchers can directly observe the location, abundance changes, and spatial relationships of target proteins at the microscopic level. Compared with traditional colorimetric or chemiluminescent detection methods, fluorescent-labeled antibodies offer clear advantages in multi-channel detection, spatial resolution, and quantitative potential, making them widely used in protein visualization and protein localization analysis.
Understanding Protein Visualization and Protein Localization Analysis
As protein research gradually shifts from qualitative assessment to spatial analysis and mechanistic studies, imaging technologies have become indispensable tools. Protein visualization and protein localization analysis represent the two core applications of fluorescent-labeled antibodies. Although they are highly similar in experimental format, they differ in research objectives, depth of data interpretation, and technical requirements. From a practical research and technical selection perspective, understanding the distinction between these two approaches helps plan experiments, choose appropriate fluorescent-labeled antibody types, and optimize imaging platforms and signal analysis strategies. Recognizing this difference is essential for fully leveraging the value of fluorescent-labeled antibody technology.
Protein Visualization: Concept and Research Objectives
Protein visualization focuses on the overall distribution and expression patterns of target proteins within samples, emphasizing the intuitive and recognizable presentation of protein signals to observe expression trends and spatial characteristics. This type of research is typically applied in protein expression validation, condition comparison studies, and preliminary functional exploration. In protein visualization studies, fluorescent-labeled antibodies are commonly used to:
- Show the presence or relative intensity of protein expression under different experimental conditions, assessing the overall impact of treatments on protein levels.
- Compare expression differences across cell types, tissue regions, or treatment groups, identifying distribution features in different biological contexts.
- Build protein expression maps or spatial schematics to provide visual references for subsequent functional studies and in-depth analysis.
Experimental designs for protein visualization prioritize signal clarity and stability, with less stringent requirements for subcellular precision or strict quantitative analysis. Its core value lies in delivering intuitive, comparable expression information.
Protein Localization Analysis: Concept and Research Objectives
Protein localization analysis focuses on the precise spatial distribution of target proteins within cells or tissues, emphasizing their relationship with cellular structures and subcellular compartments. This research is typically used to investigate protein function, regulatory mechanisms, and spatial behavior in signaling pathways. In protein localization analysis, fluorescent-labeled antibodies are commonly used to:
- Determine subcellular localization characteristics, such as enrichment in specific organelles or functional regions.
- Analyze spatial distribution changes under different treatments or stimuli.
- Evaluate spatial correlations and overlap with other markers.
Compared with protein visualization, protein localization analysis requires higher spatial accuracy and consistency of fluorescent signals. Results are often used to support mechanistic inference or functional modeling.
How Fluorescent-Labeled Antibodies Enable Protein Visualization?
Fluorescent-labeled antibodies achieve direct visualization of proteins in cells or tissues by specifically recognizing target proteins and localizing detectable fluorescent signals to the binding sites. This approach increases experimental information density and provides strong support for comparative analysis of expression patterns and spatial distribution. The application of fluorescent-labeled antibodies balances signal intensity, resolution, and reproducibility, making protein visualization more precise and efficient.
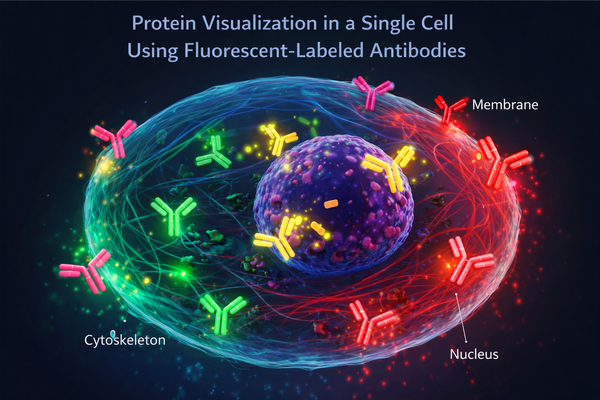 Fig. 1. Protein visualization in a single cell using fluorescent-labeled antibodies (BOC Sciences Authorized).
Fig. 1. Protein visualization in a single cell using fluorescent-labeled antibodies (BOC Sciences Authorized).
Visualization of Protein Expression Patterns
In protein visualization experiments, fluorescent-labeled antibodies display the expression regions and intensity changes of target proteins across different samples. By carefully designing experimental conditions and selecting appropriate fluorescent dyes, researchers can clearly observe protein distribution and assess the overall effects of experimental treatments. Fluorescent-labeled antibodies in this context are typically used to:
- Show spatial distribution and relative intensity under various treatment conditions, enabling evaluation of overall experimental effects.
- Compare expression patterns across cell types or tissue regions, identifying differential distribution in biological contexts.
- Build protein expression maps or visual schematics to provide foundational data for further functional research and experimental planning.
This approach emphasizes intuitive presentation and trend recognition, serving as a key tool for functional exploration and preliminary validation.
Multicolor Fluorescence Imaging for Protein Mapping
Multicolor fluorescence imaging uses antibodies labeled with different emission wavelengths to simultaneously observe multiple proteins in a single experiment. This technique is particularly advantageous for studying protein interactions, complex pathways, and spatial localization, revealing relationships among protein networks and functional modules. In multicolor imaging applications, fluorescent-labeled antibodies are typically used to:
- Detect multiple proteins simultaneously in cells or tissues, increasing experimental information density.
- Support co-expression and co-localization analysis, providing spatial evidence for protein interactions.
- Build multi-protein spatial maps to aid understanding of complex signaling pathways.
Precise matching of excitation and emission wavelengths and minimizing signal crosstalk ensure reliable and reproducible imaging results.
Fluorescent Signal Intensity and Imaging Resolution Considerations
Protein visualization experiments require balancing signal intensity and spatial resolution. Low fluorescence can obscure target signals, while excessive labeling or dye concentration can cause saturation or background interference. Selecting appropriate fluorescent-labeled antibodies and optimizing imaging parameters are key to high-quality visualization. In experimental design, fluorescent-labeled antibodies are typically used to:
- Provide stable and strong fluorescent signals to clearly observe protein distribution.
- Maintain spatial resolution and signal-to-noise ratio across different imaging platforms.
- Support quantitative or semi-quantitative analysis, providing data on expression levels and spatial distribution differences.
Through careful adjustment of labeling strategies and imaging conditions, researchers can achieve high-precision visualization and lay a solid foundation for localization analysis and functional studies.
Looking for Antibody Labeling Dyes?
We provide flexible conjugation options with various fluorophores, including water-soluble and photostable dyes, to meet your experimental requirements.
Role of Fluorescent-Labeled Antibodies in Protein Localization Analysis
Protein localization analysis focuses on the precise spatial distribution, subcellular localization, and dynamic changes of target proteins. Fluorescent-labeled antibodies, as highly specific binding tools, play a critical role in acquiring spatial information and supporting quantitative analysis. By selecting suitable dyes and optimizing antibody conjugation, researchers can achieve fine-scale localization at the microscopic level, providing direct evidence for molecular mechanisms and functional networks.
Subcellular Localization and Spatial Distribution Analysis
Fluorescent-labeled antibodies enable precise labeling of proteins within organelles or specific structures, allowing observation of subcellular distribution. Combined with organelle-specific markers, they clarify protein enrichment in different structures and provide foundational data for functional analysis. In subcellular localization studies, fluorescent-labeled antibodies are typically used to:
- Identify protein distribution in the nucleus, mitochondria, endoplasmic reticulum, or plasma membrane.
- Analyze spatial translocation or enrichment patterns under different treatments.
- Build subcellular protein distribution maps, providing visual references for functional localization and mechanistic studies.
This precise localization capability supports scientific inference and mechanistic exploration, beyond simple signal observation.
Protein Co-Localization and Pathway Investigation
Using multi-channel fluorescence imaging, fluorescent-labeled antibodies detect spatial overlap or proximity between different proteins. Co-localization information provides critical clues for signaling pathways, protein interactions, and functional modules, helping researchers understand spatial logic in complex biological processes. In co-localization analysis, fluorescent-labeled antibodies are typically used to:
- Identify spatial overlap between target proteins and other proteins or cellular structures.
- Analyze distribution patterns and trends in signaling pathways.
- Build multi-protein co-localization maps to support pathway analysis and mechanistic research.
Optimizing dye selection and signal separation ensures accuracy and reliability in multi-protein co-localization studies.
Quantitative Localization Analysis Using Fluorescent Signals
Beyond visualizing protein distribution, fluorescent-labeled antibodies support quantitative or semi-quantitative spatial analysis. Standardizing imaging conditions and signal intensity analysis enables comparison of protein localization across experimental groups or time points, providing measurable data. In quantitative localization studies, fluorescent-labeled antibodies are typically used to:
- Deliver stable, reproducible signals for numerical analysis of spatial distribution.
- Compare protein enrichment intensity under different conditions and perform statistical evaluation.
- Support construction of dynamic protein distribution models, revealing movement and translocation patterns in cellular processes.
Combining quantitative analysis with spatial precision allows protein localization studies to generate statistically robust data, providing a strong foundation for in-depth functional and mechanistic research.
Common Applications of Fluorescent-Labeled Antibodies
Fluorescent-labeled antibodies are widely used in cell and molecular biology research due to their high specificity, direct imaging capability, and compatibility with multi-channel detection. Their applications span protein expression analysis, subcellular localization, signaling pathway investigation, and dynamic protein behavior studies, making them indispensable tools in modern protein research. By selecting appropriate fluorescent dyes and optimizing labeling strategies, researchers can obtain high-quality spatial and functional information across different experimental systems.
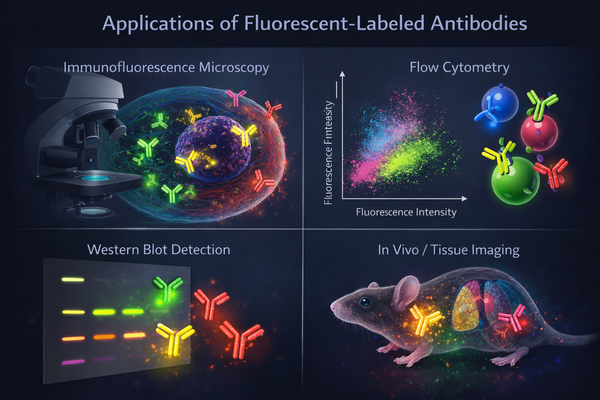 Fig. 2. Applications of fluorescent-labeled antibodies across immunofluorescence microscopy, flow cytometry, Western blot analysis, and tissue imaging (BOC Sciences Authorized).
Fig. 2. Applications of fluorescent-labeled antibodies across immunofluorescence microscopy, flow cytometry, Western blot analysis, and tissue imaging (BOC Sciences Authorized).
Immunofluorescence-Based Cell and Tissue Imaging
Immunofluorescence represents one of the most classic applications of fluorescent-labeled antibodies and can be used for fixed cells, tissue sections, and various biological samples. Coupled with fluorescence microscopy, researchers can observe protein distribution patterns and expression characteristics, providing intuitive visual data for functional studies. In this application, fluorescent-labeled antibodies are typically used to:
- Display differences in protein distribution across cell types or tissue regions.
- Construct spatial protein expression maps to provide foundational data for functional and mechanistic studies.
- Support multicolor fluorescence imaging for simultaneous detection of multiple proteins and analysis of spatial relationships.
This approach balances experimental intuitiveness with data reliability, making it a key tool for protein function validation and basic research.
Protein Trafficking and Dynamic Localization Studies
Protein trafficking and dynamic localization within cells are critical for understanding signal transduction, metabolic regulation, and molecular mechanisms. Fluorescent-labeled antibodies allow real-time or time-lapse imaging of spatial changes under different conditions, providing direct evidence for dynamic behavior studies. In dynamic localization research, fluorescent-labeled antibodies are typically used to:
- Track the entire process of protein synthesis, transport, and final localization.
- Analyze translocation and enrichment patterns under different stimuli or treatment conditions.
- Construct dynamic protein distribution models to reveal regulatory mechanisms in cellular processes.
By combining time-lapse imaging and high-resolution microscopy, researchers can gain deep insights into the spatial and temporal characteristics of protein behavior.
Fixed-Cell and Live-Cell Protein Analysis
Fluorescent-labeled antibodies can be applied in both fixed and live-cell systems to meet different experimental needs. In fixed cells, antibody labeling provides high-resolution spatial information suitable for subcellular localization and multi-channel imaging. In live cells, specially designed fluorescent antibodies can preserve protein functionality for real-time dynamic observation. In both applications, fluorescent-labeled antibodies are typically used to:
- Perform high-precision imaging of protein spatial distribution and enrichment within cells.
- Observe protein movement and translocation in live-cell environments.
- Support multi-channel co-imaging for analyzing spatial relationships and dynamic interactions between proteins.
This flexibility allows fluorescent-labeled antibodies to support a range of studies, from basic expression analysis to complex functional mechanism investigation.
Key Considerations When Selecting Fluorescent-Labeled Antibodies
Proper selection of fluorescent-labeled antibodies is critical for ensuring experimental success and data reliability. Different research objectives, imaging platforms, and sample types impose distinct requirements on antibody properties and fluorophore performance. Scientific selection enhances signal specificity and stability while optimizing experimental efficiency and imaging quality.
Fluorophore Selection for Different Imaging Platforms
Fluorescent dyes must match the excitation and emission characteristics of imaging systems to ensure signal separation and sensitivity in multi-channel imaging. Different platforms have specific requirements for dye spectra, photostability, and signal-to-noise ratio. Key considerations in fluorophore selection include:
- Compatibility of excitation/emission wavelengths with microscope filters and detectors.
- Photostability and anti-photobleaching performance for long-term or dynamic imaging.
- Spectral separation in multicolor experiments to avoid signal crosstalk.
- Choosing single-channel or multi-channel dye combinations to maximize information acquisition.
Careful fluorophore selection enables high-quality, reproducible protein visualization and localization analysis across different experimental systems.
Antibody Specificity and Target Compatibility
High-specificity antibodies are essential for reliable signals and low background noise. Antibody binding efficiency, epitope recognition, and cross-reactivity directly affect imaging quality and localization accuracy. Selection considerations include:
- Whether the antibody targets native or modified epitopes of the protein.
- Binding efficiency in different sample types (cells, tissues, or secretions).
- Non-specific binding that may generate background signals.
- Batch-to-batch consistency to ensure reproducibility in long-term studies.
High-specificity fluorescent-labeled antibodies improve signal quality and ensure scientifically reliable and interpretable results.
Photostability, Signal-to-Noise Ratio, and Multiplexing Needs
Fluorescent signal stability and clarity directly influence imaging accuracy and data reliability. For long-term observations or multi-channel analyses, selecting photostable antibodies with high signal-to-noise ratios is critical. Multiplexed experiments require compatible dye combinations to maintain independent, analyzable signals. In these applications, fluorescent-labeled antibodies are typically used to:
- Provide persistent and stable signals for time-lapse or dynamic imaging.
- Ensure high signal-to-noise ratios for precise spatial distribution and co-localization analysis.
- Support multi-channel labeling and co-imaging to fully analyze spatial relationships between proteins.
- Minimize photobleaching and crosstalk to guarantee reproducible and comparable data.
Scientific selection of antibodies and dye combinations improves experimental efficiency, reduces repetition, and ensures high-quality spatial information.
Technical Challenges in Fluorescent Antibody-Based Analysis
Despite their advantages in protein visualization and localization analysis, fluorescent-labeled antibodies face technical challenges that can affect accuracy, reproducibility, and interpretability. Understanding and addressing these issues is critical for successful and scientifically robust experiments.
Background Fluorescence and Non-Specific Binding
Background fluorescence and non-specific binding are common sources of interference. Autofluorescence from samples or non-specific antibody binding can obscure target signals. Non-specific interactions between dyes and samples can increase background and reduce signal-to-noise ratio, affecting spatial resolution. Optimizing antibody concentration, blocking steps, and washing conditions is essential to minimize background and obtain reliable imaging results.
Fluorophore Quenching and Signal Stability
Fluorescent signals may be quenched by light exposure, chemical environment, or dye properties, leading to intensity loss or uneven signals. This is particularly problematic in long-term imaging, dynamic observation, or multi-channel experiments. Selecting photostable dyes with strong anti-photobleaching properties and controlling imaging conditions are key to maintaining reliable results.
Reproducibility and Batch Consistency Issues
Differences in labeling density, binding efficiency, and dye activity across antibody batches or sources may affect long-term or cross-experiment comparability. Ensuring batch consistency and antibody quality stability is fundamental for reproducibility. Standardizing labeling procedures and imaging parameters also reduces experimental bias and improves data comparability.
Custom Fluorescent Antibody Labeling Solutions at BOC Sciences
BOC Sciences offers comprehensive custom fluorescent antibody labeling services to meet diverse protein research needs. Whether for protein visualization, subcellular localization, or multiplexed analysis, we provide high-quality conjugation solutions that ensure stable, specific fluorescent signals while maintaining reproducibility and imaging precision. Our professional team and systematic workflow support full-process solutions, from antibody selection and dye conjugation to final validation, accelerating research progress.
Customized Fluorophore Selection for High-Precision Imaging
- Offers a wide range of fluorescent dyes, including FITC (Fluorescein Isothiocyanate), Alexa Fluor series, DyLight series, Rhodamine, Cy3/Cy5, and long-wavelength near-infrared dyes, covering visible to near-infrared spectra.
- Optimizes dye combinations based on microscope configuration, excitation sources, and multi-channel imaging needs to minimize spectral overlap and crosstalk.
- Provides dye conjugation density control to balance antibody activity and fluorescence intensity.
- Selects photostable dyes for live-cell and fixed-cell experiments to ensure stable signals during long-term or dynamic imaging.
Advanced Antibody Conjugation Techniques to Maintain Specificity
- Employs established conjugation chemistries, including NHS ester, thiol-based, and click chemistry, to ensure stable dye-antibody binding without compromising antibody activity.
- Compatible with various antibody types, including monoclonal, polyclonal, and fragments (Fab/ScFv), providing customized solutions for diverse experimental needs.
- Optimizes labeling density to prevent photobleaching and non-specific background while maintaining imaging resolution and signal-to-noise ratio.
- Offers post-conjugation antibody validation, including binding activity tests, fluorescence intensity measurement, and batch consistency assessment to ensure reproducibility.
Tailored Solutions for Specific Protein Research Applications
- Designs labeling strategies for protein visualization, ensuring clear signals and accurate distribution for comparative analysis under different conditions.
- Provides multi-channel, multicolor labeling for subcellular localization and co-localization studies, with spectral optimization for precise spatial resolution.
- Selects photostable, low-toxicity dyes and optimized conjugation ratios for dynamic protein behavior studies and live-cell imaging to preserve protein function.
- Offers technical consultation and experimental optimization, including antibody dilution strategies, labeling conditions, imaging parameter guidance, and data interpretability assessment.
Comprehensive Quality Assurance and Customer Support
- Full quality control covering antibody purity, labeling efficiency, and fluorescent signal stability to ensure reliable product performance.
- Batch-to-batch consistency guarantees reproducibility for long-term studies or cross-experiment comparisons.
- Provides detailed technical reports including dye selection, conjugation methods, fluorescence intensity, and application recommendations to support rapid experiment setup.
- Professional technical team available to respond to customer needs, supporting both small-scale experiments and large-scale production with controlled delivery timelines.
Do You Need A Consultation?
BOC Sciences integrates cutting-edge fluorescence technologies to accelerate your research, driving next-generation solutions for drug discovery and diagnostics.
Transform Your Studies with Cutting-Edge Fluorescent Products
| Cat. No. | Product Name | CAS No. | Inquiry |
|---|---|---|---|
| R01-0009 | BDY FL, SE | 146616-66-2 | Bulk Inquiry |
| A16-0170 | Rhodamine-123 | 62669-70-9 | Bulk Inquiry |
| A19-0040 | Hoechst 33342 | 23491-52-3 | Bulk Inquiry |
| A19-0103 | SYBR Green I | 163795-75-3 | Bulk Inquiry |
| A14-0036 | Rhodamine B hydrazide | 74317-53-6 | Bulk Inquiry |
| F06-0011 | Coumarin 153 | 53518-18-6 | Bulk Inquiry |
| A17-0009 | LDS 867 | 106025-71-2 | Bulk Inquiry |
| F02-0026 | Cy5-NHS ester | 146368-14-1 | Bulk Inquiry |
| F01-0251 | BODIPY 576/589 | 150173-78-7 | Bulk Inquiry |
| F02-0007 | Cyanine5 amine | 1807529-70-9 | Bulk Inquiry |
High-Performance Fluorescent Tools for Your Research
- TAMRA DyesRed-emitting dyes for antibody and protein labeling.
- ATTO DyesHigh-performance dyes for labeling and imaging experiments.
- CoumarinBlue-emitting dyes for chemical sensing and fluorescence studies.
- Fluorescent ProteinUsed for live-cell imaging and real-time biosensing.
More About Antibody labeling
Online Inquiry

