BODIPY
Background
BODIPY dyes are a unique class of fluorescent dyes known for their high quantum yields, exceptional photostability, and tunable spectral properties. They are widely used in bioimaging, fluorescent probes, sensors, and optoelectronic materials. BOC Sciences offers a diverse range of BODIPY dyes to meet the needs of both basic research and industrial applications. Leveraging advanced synthesis technologies and custom services, we provide high-purity, functionalized BODIPY derivatives with options for bulk production, structural modification, and performance optimization—empowering innovation in drug development, materials science, and biological detection.
What is BODIPY Dye?
Boron-dipyrromethene (BODIPY) dyes are a class of near-infrared fluorescent dyes with excellent performance. Its molecular structure has a larger conjugated system, as well as greater planarity and rigidity. By modifying the molecular structure of BODIPY, a series of derivatives could be obtained for applications in life sciences, environmental monitoring and other fields.
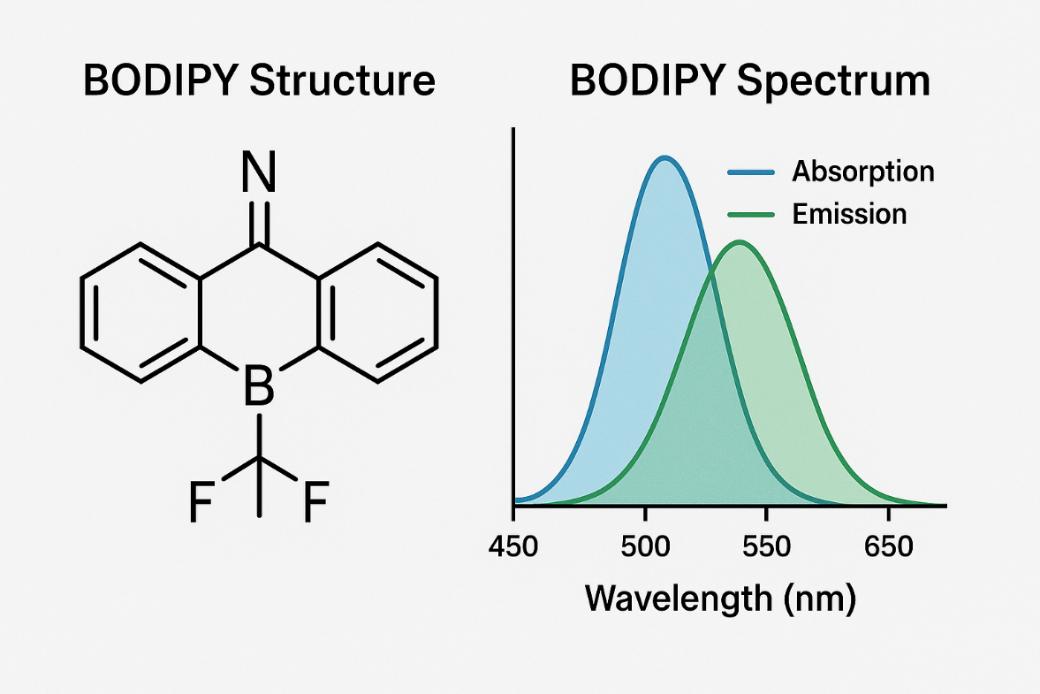 Fig. 1. BODIPY structure and
BODIPY spectrum (BOC Sciences Authorized).
Fig. 1. BODIPY structure and
BODIPY spectrum (BOC Sciences Authorized).
BODIPY Structure and Chemical Features
BODIPY dyes are a class of fluorescent dyes with a unique chemical structure. Their core structure consists of a boron-dipyrromethene moiety, which gives BODIPY dyes excellent optical properties. The molecular weight of BODIPY dyes typically ranges from 500 to 1000 Daltons, depending on the modification of their side chains. The symmetry and conjugated system of their molecular structure allow them to exhibit high brightness and low phototoxicity in fluorescence imaging, making them highly suitable for a wide range of biological applications, including live-cell imaging, lipid and protein labeling, and multicolor fluorescence experiments.
BODIPY Solubility and Stability Considerations
The physicochemical properties of BODIPY dyes significantly influence their applications, with solubility and stability being key factors in experimental design. Most BODIPY dyes are well soluble in organic solvents such as dimethyl sulfoxide (DMSO) and ethanol. Through chemical modification, however, water-soluble derivatives can also be synthesized, expanding their applicability in biomedical research. Furthermore, the molecular structure of BODIPY is closely related to its fluorescence properties and stability. By adjusting side-chain length and functional groups, the dye's emission wavelength, quantum yield, and photochemical stability can be optimized to meet the requirements of high-performance labeling under diverse experimental conditions.
Benefits of BODIPY Dyes in Science
Compared with rhodamine or cyanine dyes, BODIPY dyes offer several superior properties:
- High molar extinction coefficient and strong light sensitivity;
- High fluorescence quantum yield (usually >0.6) with low self-quenching;
- Stable spectral properties, minimally affected by pH and solvent polarity;
- Narrow emission peak, high detection sensitivity;
- Excellent photostability, thermal, and chemical stability;
- Low molecular weight and cytotoxicity;
- Easily modifiable molecular structure;
- Long excitation wavelength, typically in the near-infrared region.
Unlocking the Excitation and Emission Spectrum of BODIPY
The optical properties of BODIPY dyes form the basis for their wide application in biomedical research. The absorption and emission spectra of BODIPY dyes usually fall within the visible light range, enabling their detection under conventional fluorescence microscopes. For example, BODIPY 493/503 is a commonly used green fluorescent dye with a maximum absorption wavelength of 493 nm and a maximum emission wavelength of 503 nm. Due to its good water solubility and low cytotoxicity, this dye is widely used for cell imaging and lipid droplet staining. In addition, BODIPY dyes have high fluorescence intensity and excellent photostability, ensuring that their fluorescence signal does not significantly decay even under prolonged illumination.
| Dye Name | Excitation (nm) | Emission (nm) | Notes |
| BODIPY 493/503 | 493 | 503 | Commonly used for lipid droplet staining |
| BODIPY 581/591 C11 | 581 (reduced) / 500–510 (oxidized) | 591 (reduced) / 510–520 (oxidized) | Emission shifts with oxidation state |
| BODIPY 488 | 503 | 512 | Similar to FITC, excitable by 488 nm laser |
| BODIPY 493 | 493 | 503 | Similar to BODIPY 493/503 |
| BODIPY 500/510 | 500 | 510 | Short-wavelength green fluorescent dye |
| BODIPY 505/515 | 505 | 515 | Common for neutral lipid staining (e.g., lipid droplets) |
| BODIPY 558/568 | 558 | 568 | Orange-red fluorescent dye |
| BODIPY 576/589 | 576 | 589 | Strong lipid-binding affinity |
| BODIPY 581/591 | 581 | 591 | Similar to C11, used to monitor oxidation |
| BODIPY 630/650 | 630 | 650 | Deep red fluorescence, suitable for multiplexing |
| BODIPY 650/665 | 650 | 665 | Near-infrared region, suitable for deep tissue imaging |
| BODIPY 665/676 | 665 | 676 | High tissue penetration, suitable for in vivo imaging |
| BODIPY C11 | ~581 | ~591 | Oxidation-sensitive, used for lipid peroxidation detection |
| BODIPY C12 | 500–510 | 510–520 | Used in lipid metabolism studies |
| BODIPY C16 | ~500–510 | ~510–520 | Long-chain fatty acid probe, used for tracking lipid metabolism |
Table 1. Absorption and emission spectral data of common BODIPY dyes. (Please note: Different suppliers may use slightly different chemical structures or labeling systems for dyes with the same name. Therefore, the exact absorption/emission peaks may vary by ±2–5 nm. It is recommended to consult the specific product datasheet before use.)
BODIPY Synthesis and Design for High-Performance BODIPY Dyes
The synthesis of specific BODIPY derivatives according to experimental requirements is an important research direction. Researchers can design and synthesize BODIPY labels with specific affinities based on the properties of target biomolecules. The design of high-performance BODIPY dyes should follow several key principles. First, the stability of the core conjugated system must be maintained to ensure the dye's optical properties and fluorescence intensity. Second, large-scale molecular aggregation should be avoided to minimize fluorescence quenching, thereby preserving signal reliability and reproducibility. Finally, hydrophilic or hydrophobic derivatives should be selected according to the experimental environment to optimize dye solubility and distribution in different media, enabling the design of BODIPY dyes that are bright, stable, and suitable for a wide range of bioimaging and fluorescence labeling applications. This customized synthesis strategy not only improves experimental specificity but also provides new ideas for the development of novel biological probes. The synthesis of BODIPY dyes typically involves constructing the core structure—the dipyrromethene scaffold—followed by coordination with boron trifluoride (BF₃·OEt₂) to form a stable BODIPY structure. Typical synthesis steps include:
- Synthesis of pyrrole derivatives: Functionalized pyrrole monomers are obtained through Fischer indole synthesis or other organic synthesis strategies.
- Formation of dipyrromethene: Two pyrrole monomers are condensed through aldehyde or ketone intermediates to form dipyrromethene.
- Oxidation and complexation: The intermediate is oxidized (e.g., using DDQ) to form dipyrromethene, followed by complexation with BF₃·OEt₂ to yield the typical fluorescent BODIPY core.
Structural Modifications of BODIPY to Tune Optical Properties
In addition, structural diversification can also be achieved by introducing side chains or functionalizing the scaffold sites. For example, introducing alkyl, aryl, or hydrophilic groups at the 3,5 or 8 positions of BODIPY can modulate its solubility, fluorescence properties, or targeting capabilities. Notably, Aza-BODIPY is an important class of BODIPY derivatives in which some carbon atoms are replaced by nitrogen atoms. This structural change significantly alters the spectral properties, especially enhancing absorption and emission in the near-infrared (NIR) region, making them valuable for deep-tissue imaging and photothermal therapy. Aza-BODIPY also shows high photostability and environmental sensitivity, making it suitable for constructing multifunctional responsive fluorescent probes.
Core Substitution
Introducing electron-donating or electron-withdrawing substituents at key positions of the BODIPY core can effectively tune the molecule's absorption and emission wavelengths. This modification not only enables red-shift or blue-shift but also influences fluorescence quantum yield and Stokes shift, making the dye more adaptable to diverse experimental needs.
Side Chain Modification
By incorporating hydrophilic or hydrophobic groups into the molecular side chains, the solubility and biocompatibility of BODIPY can be optimized. Side chain modification can also enhance membrane penetration or lipid-binding ability, while increasing molecular rigidity to reduce self-aggregation and improve fluorescence stability.
π-Conjugation Extension
Extending the conjugated chain at the core or pyrrole rings can red-shift the emission wavelength into the red or near-infrared region, while simultaneously improving fluorescence quantum yield. This strategy is particularly suitable for deep tissue imaging or multicolor fluorescence experiments requiring high brightness.
Steric Hindrance Groups
The introduction of bulky steric hindrance groups can effectively prevent intermolecular aggregation, thereby reducing fluorescence quenching. This approach contributes to improved optical stability of the dye, ensuring more reliable experimental outcomes.
Isomer and Ring Fusion
Designing isomeric BODIPY structures or introducing fused-ring systems can enhance both photostability and chemical stability of the dyes. Structural isomerization can also alter spectral properties, providing broader options for multicolor fluorescence labeling.
Hydrophilic or Hydrophobic Modifications
By adjusting hydrophilic or hydrophobic groups, BODIPY dyes can maintain good solubility in aqueous solutions or lipid environments. This strategy helps the dyes perform optimally in intracellular experiments or membrane imaging applications.
Looking for Expert Guidance?
BOC Sciences harnesses cutting-edge BODIPY dye technologies to accelerate your research and drive innovation in imaging, sensing, and diagnostics.
Common BODIPY Derivatives and Their Functions
Developing structurally diverse and functionally rich BODIPY derivatives is a key direction to promote their wide applications. By introducing various functional or bioactive groups onto the BODIPY scaffold, a series of specialized molecules can be obtained for applications in biomarking, drug delivery, sensing, and energy materials. Examples include:
BODIPY-Cholesterol
Incorporation of a cholesterol group allows the derivative to insert selectively into the lipid bilayer of cell membranes via hydrophobic interactions, enabling selective membrane labeling widely used in membrane dynamics studies and visualization.
BODIPY Amine
A BODIPY molecule containing a primary amine (–NH₂) group, providing a reactive site for chemical modification and covalent conjugation with proteins or small molecules, expanding its use in fluorescent probes and biological labeling.
BODIPY Azide
A BODIPY derivative with an azide (–N₃) group that participates in highly efficient and specific click chemistry reactions (e.g., CuAAC), widely used for biomolecule labeling, material modification, and complex molecule construction.
BODIPY Carboxylic Acid
A BODIPY molecule bearing a carboxyl (–COOH) group that enables covalent linkage via amide bonds to amine-containing molecules like proteins and antibodies, commonly applied in bioimaging and functional probe design.
BODIPY Ceramide
A derivative where BODIPY is attached to ceramide, a key lipid in cell membranes and signaling, allowing visualization of lipid distribution and metabolic dynamics within cells.
BODIPY Cyclopamine
A BODIPY-labeled derivative of cyclopamine, a Hedgehog pathway inhibitor, combining pharmacological activity and fluorescence for cellular studies of distribution and targeting.
BODIPY Fatty Acid
BODIPY conjugated with fatty acid chains of various lengths, exhibiting lipophilicity that facilitates insertion into membranes or lipid droplets for studies of lipid metabolism, uptake, transport, and imaging.
BODIPY Maleimide
A BODIPY derivative containing a maleimide group that reacts specifically with thiol (–SH) groups such as cysteine residues, commonly used for labeling proteins, peptides, and sulfur-containing biomolecules.
BODIPY NHS Ester
A BODIPY derivative with an active N-hydroxysuccinimide (NHS) ester group that reacts with primary amines to form stable amide bonds, widely used for fluorescent labeling of proteins and other amine-containing molecules in biochemical research.
BODIPY Tetrazine
A BODIPY dye linked to tetrazine, enabling fast, selective bioorthogonal click reactions with strained alkenes for precise fluorescent labeling and real-time imaging of biomolecules in live cells.
Understanding Fluorescence Quenching Mechanisms in BODIPY Dyes
Fluorescence quenching is a common issue in experiments involving BODIPY dyes, leading to reduced signal intensity and data instability. Quenching may arise from intrinsic effects caused by intermolecular interactions, or from external factors such as oxygen, metal ions, or solvents. Experimental conditions including temperature, pH, solvent polarity, and molecular aggregation significantly affect the luminescence efficiency of the dyes. To mitigate quenching, bulky steric hindrance groups can be introduced to prevent molecular aggregation, while solution conditions can be optimized, and stable derivatives or reinforced conjugated systems can be selected to enhance photochemical stability. Rational control of environmental factors combined with molecular design strategies can greatly improve the signal intensity and reliability of BODIPY in diverse fluorescence applications such as protein, nucleic acid, lipid, and live-cell imaging, ensuring stable and reproducible results that support both scientific and industrial applications.
Enhancing Fluorescent Labeling Performance with BODIPY Dyes
The flexible chemical structure of BODIPY allows the dye to adapt to the requirements of various biomolecules and experimental conditions. Whether used with proteins, peptides, nucleic acids, lipids, or membrane systems, BODIPY enables efficient and specific fluorescent labeling. Furthermore, its multifunctional derivatives support multicolor labeling, live-cell imaging, and environment-responsive probe design, ensuring stable signal intensity with minimal background interference. By carefully selecting labeling strategies and chemical conjugation methods, researchers can maximize fluorescence signals while maintaining the functional integrity of biomolecules, resulting in more reliable and reproducible outcomes. These advantages make BODIPY an indispensable tool for modern fluorescence experiments, offering wide-ranging possibilities for both research and industrial applications.
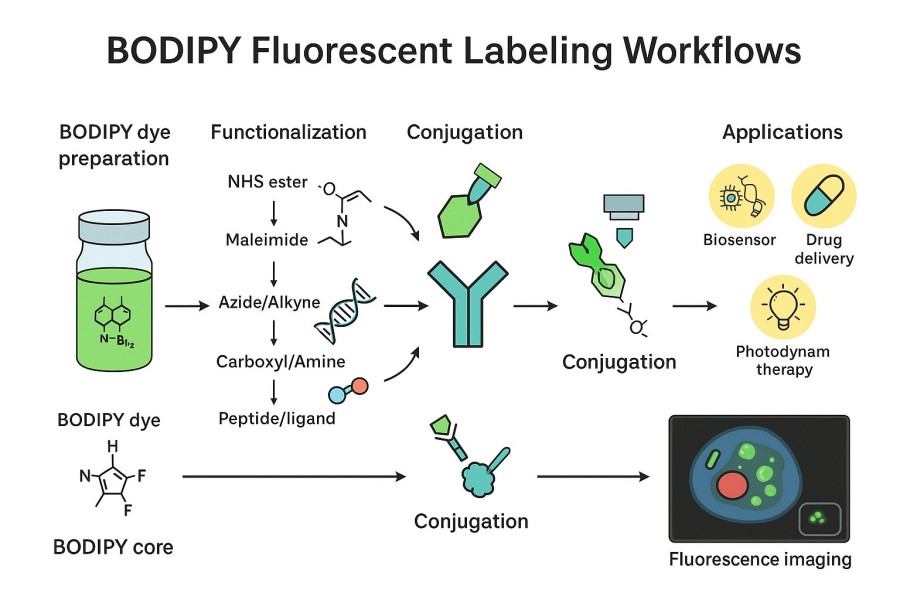
- Protein and Peptide Covalent Labeling: Stable covalent bonds are formed with proteins or peptides via amino or thiol groups, achieving highly specific fluorescent labeling while preserving biomolecular functions.
- Lipid and Membrane System Embedding: Hydrophobic side chains or lipid-binding groups allow BODIPY to embed into cell membranes or liposomes, useful for membrane imaging, lipid metabolism tracking, and membrane dynamics studies.
- Nucleic Acid Covalent Conjugation: BODIPY can be conjugated to DNA or RNA through phosphate ester bonds, terminal modifications, or click chemistry methods, enabling efficient and stable fluorescent nucleic acid labeling.
- Click Chemistry Labeling: Using CuAAC or SPAAC click reactions, BODIPY can be specifically linked to target molecules under mild conditions, improving labeling efficiency and biocompatibility.
- Multicolor Live-Cell Imaging: Different BODIPY derivatives can be combined for multicolor labeling with high photostability and low quenching, enabling dynamic live-cell observation and signal pathway analysis.
Enhance Your Research with Efficient BODIPY Staining
To achieve reliable and efficient applications, understanding the bodipy staining principle is essential. It reveals the binding mechanisms of BODIPY dyes with biomolecules such as proteins, lipids, or nucleic acids, as well as their role in fluorescence signal generation. At the same time, mastering a standardized bodipy staining protocol ensures experimental reproducibility and high-quality results. From sample preparation to dye concentration selection and imaging condition optimization, each step directly influences final signal intensity and stability. By combining scientific understanding of principles with adherence to standardized procedures, researchers can fully harness the advantages of BODIPY in cell imaging, flow cytometry, and molecular labeling, thereby improving experimental efficiency and the reliability of scientific outcomes. Below are some representative bodipy staining protocols:
BODIPY Lipid Droplet Staining Protocol
Materials and Reagents
- Cell line: 3T3-L1 adipocytes;
- BODIPY dye: BODIPY 493/503;
- Other reagents: DMEM medium, insulin, dexamethasone, triglycerides.
Staining Steps and Results
- Cell culture: Culture 3T3-L1 cells in DMEM to 80% confluency;
- Differentiation induction: Add insulin, dexamethasone, and triglycerides to induce adipocyte differentiation;
- Staining: Wash differentiated cells with PBS and incubate with 1 μM BODIPY 493/503 for 30 minutes;
- Microscopy: Observe lipid droplet distribution under a fluorescence microscope. Results show clear visualization of droplet size and number with stable signal.
BODIPY Live Cell Imaging Protocol
Experimental Design and Cell Culture
- Cell line: HeLa cells;
- BODIPY dye: BODIPY-cholesterol;
- Other reagents: DMEM medium, fetal bovine serum.
Dye Labeling and Fluorescence Imaging
- Cell culture: Grow HeLa cells to 70% confluency in DMEM;
- Dye labeling: Wash cells with PBS, then incubate with 2 μM BODIPY-cholesterol for 15 minutes;
- Microscopy: Observe membrane fluorescence using live-cell imaging. Results show specific labeling of the cell membrane and clear visualization of its structure.
BODIPY Staining Tissue Protocol
Tissue Section Preparation
- Tissue: Mouse adipose tissue;
- Fixative: 4% formaldehyde;
- Embedding medium: Paraffin.
BODIPY Staining and Microscopy
- Fixation and embedding: Fix tissue in 4% formaldehyde for 24 hours, then embed in paraffin;
- Sectioning and staining: Deparaffinize 5 μm tissue sections and incubate with 1 μM BODIPY 493/503 for 30 minutes;
- Microscopy: Use a fluorescence microscope to observe lipid droplet distribution. Results show clear structures of lipid droplets in adipocytes.
Cutting-Edge BODIPY Applications in Research and Industry
BODIPY-based ion and molecular probes can detect various inorganic or organic ions and molecules with high efficiency and sensitivity. BODIPY dyes are also widely used as fluorescent markers in protein detection, nucleic acid hybridization sequencing, and disease diagnostics. Additionally, functionalized BODIPY dyes can generate singlet oxygen, showing potential as photosensitizers in photodynamic therapy. Beyond biochemical labeling, BODIPY dyes are also used in lasers, dye-sensitized solar cells, and light-trapping systems.
BODIPY Lipid Droplet Staining
Lipid droplets are key organelles for intracellular lipid storage. BODIPY dyes have a unique advantage in staining lipid droplets by selectively binding to the lipid bilayer. Common dyes include BODIPY 493/503 and BODIPY 550/565. These dyes not only reveal lipid droplet distribution but also indicate droplet size and number via fluorescence intensity changes. For example, during lipid metabolism research, BODIPY dyes can be used to observe droplet formation, fusion, and degradation.
BODIPY for Lipid Staining
BODIPY dyes can serve as lipid probes in lipid metabolism research. By chemical modification, BODIPY lipid probes with specific affinity can be synthesized. These probes bind selectively to lipid molecules and can be detected under microscopy. For instance, during lipid transport studies, BODIPY lipid probes track intracellular lipid dynamics. They are also useful for investigating lipid-protein interactions, revealing molecular mechanisms of lipid metabolism.
Live Cell Imaging with BODIPY Dyes
BODIPY dyes are widely used in cell imaging due to low phototoxicity and good biocompatibility, making them ideal for live-cell applications. In flow cytometry, BODIPY dyes assist in cell sorting and cell cycle analysis. For instance, by labeling cell membranes with BODIPY dyes, rapid cell sorting can be achieved. BODIPY dyes also enable visualization of dynamic cellular processes, such as in cell migration assays where cell membranes are labeled to track movement.
BODIPY Staining for Live and Fixed Cells
BODIPY staining is suitable for both fixed and live-cell imaging. Fixed cells are typically preserved with formaldehyde to maintain structural stability, followed by incubation with an appropriate concentration of BODIPY dye. For example, BODIPY 493/503 staining of lipid droplets requires about 30 minutes, and conditions should be optimized to avoid over- or under-staining. Live-cell staining relies on the dye's low phototoxicity and excellent compatibility, usually entering cells by passive diffusion and binding to lipid structures to achieve clear subcellular imaging.
BODIPY Dyes in Drug Delivery
Due to their excellent photostability, narrow emission peaks, and tunable chemical modifications, BODIPY dyes are widely used in drug delivery research. They can label nanocarriers, liposomes, or drug molecules, enabling highly sensitive tracking of drug distribution and release processes in vitro and in vivo. Through controlled derivatization design, BODIPY dyes can form covalent bonds with drugs or carriers while maintaining fluorescence signals and biocompatibility, providing a reliable tool for studying targeted drug delivery and release mechanisms.
Drug Screening with BODIPY Labels
In high-throughput drug screening, BODIPY dyes are widely applied due to their high quantum yield and low background signal. Researchers can label small molecules, proteins, or cell systems with BODIPY to rapidly assess the binding efficiency or inhibitory activity of candidate drugs against biological targets. The optical properties of BODIPY allow multicolor detection and dynamic monitoring, providing high sensitivity and reproducibility in drug screening while minimizing experimental error and data interference.
BODIPY Dyes for Flow Cytometry
BODIPY dyes perform exceptionally well in flow cytometry, with their narrow emission spectra and high fluorescence intensity supporting multicolor labeling experiments. Researchers can use BODIPY to label specific cell populations or surface markers, enabling precise cell classification, phenotyping, and functional analysis. Their excellent photostability ensures signal consistency during long-term analysis while reducing photobleaching and spectral overlap, making BODIPY a reliable fluorescence tool for cell analysis and immunophenotyping studies.
Cell Tracking with BODIPY Dyes
BODIPY dyes can be used for labeling live cells, enabling long-term tracking and migration studies. Their high photostability and low toxicity allow labeled cells to maintain normal physiological states during in vitro culture or in vivo experiments. By optimizing labeling concentrations and modification strategies, BODIPY dyes can clearly reveal cell migration pathways and distribution, providing reliable dynamic imaging tools for stem cell research, cancer metastasis studies, and tissue engineering applications.
BODIPY Dyes in Molecular Diagnostics
BODIPY derivatives are widely used in molecular diagnostics, particularly in the design of fluorescent probes and sensors. They can specifically bind to biomolecules such as DNA, RNA, and proteins, enabling rapid and sensitive detection. Leveraging BODIPY's optical advantages, researchers can achieve signal amplification and multicolor detection even in complex samples while minimizing background interference. These characteristics make BODIPY highly valuable in genetic testing, early disease screening, and biomarker analysis.
BODIPY Molecular Probe Design
BODIPY dyes are extensively used in fluorescent probe design due to their outstanding optical properties and chemical modifiability. Through core or side-chain functionalization, highly specific detection of target biomolecules or environmental factors such as pH, metal ions, or lipid changes can be achieved. Combined with covalent bonding or click chemistry strategies, BODIPY probes can stably label proteins, nucleic acids, and cell membrane structures, enabling live-cell imaging, multicolor labeling, and dynamic monitoring. These features provide reliable tools for molecular imaging and biosignal research.
Frequently Asked Questions
-
What does BODIPY stain?
BODIPY dyes are high-performance fluorescent probes capable of selectively labeling various cellular components and biomolecules. They are commonly used to stain lipid droplets, cell membranes, proteins, and nucleic acids, with extensive applications in lipid metabolism and membrane structure studies. With high photostability, strong fluorescence intensity, and good cell compatibility, BODIPY dyes clearly reveal intracellular structures and molecular distributions, helping researchers visualize and analyze diverse cellular processes.
-
How does BODIPY staining work?
BODIPY staining relies on the fluorophore's optical properties and affinity for target molecules. When excited at specific wavelengths, the dye emits strong and stable fluorescence and selectively binds to cellular components such as lipids or proteins. In cell experiments, BODIPY typically enters cells via passive diffusion or forms complexes with target molecules to achieve specific labeling. Low phototoxicity and high stability ensure minimal interference with cell activity, making BODIPY suitable for both fixed and live-cell imaging.
-
How to do BODIPY staining?
The basic steps of BODIPY staining include cell preparation, dye incubation, and microscopic observation. Fixed cells are usually treated with formaldehyde to preserve structure, followed by incubation with an appropriate concentration of BODIPY dye for a certain period. Live-cell staining requires maintaining cell viability, with the dye entering cells via diffusion and binding to lipids or other targets. After incubation, excess dye is removed with buffer, and imaging is performed under a fluorescence microscope. Experimental parameters such as dye concentration and incubation time should be optimized based on research goals to obtain clear and reliable fluorescence images.
-
What are the advantages of BODIPY?
BODIPY dyes offer multiple advantages. First, they exhibit strong fluorescence intensity with clear signals and minimal background interference. Second, they possess excellent photostability, maintaining signals during long-term imaging. Third, their low phototoxicity and high cell compatibility make them ideal for live-cell imaging. Finally, their stable chemical structure allows for modification to achieve diverse spectral properties and biological functions. These advantages make BODIPY widely applicable in probe design, fluorescence labeling, and bioimaging.
-
What is BODIPY used for?
BODIPY is widely used in both biological and chemical research. Its primary applications include fluorescent labeling of cell membranes, lipid droplets, proteins, and nucleic acids for cellular imaging and subcellular structure studies. In drug development, BODIPY is often employed as a molecular probe to investigate drug distribution and mechanisms of action. It is also used in flow cytometry, live-cell tracking, molecular diagnostics, and high-throughput drug screening. With superior optical performance and strong modifiability, BODIPY has become an important fluorescent dye in both research and industrial applications.
-
What is the BODIPY stain for lipids?
BODIPY dyes are commonly applied in lipid-related studies, particularly in staining lipid droplets and membrane lipids. For instance, BODIPY 493/503 selectively labels neutral lipid droplets, revealing their distribution and dynamic changes within cells. During staining, dye molecules interact with the bilayer structure of lipid droplets, emitting strong and stable fluorescence signals. This method not only visualizes lipid localization in cells but also facilitates studies of lipid metabolism, energy storage, and related disease mechanisms, making it a vital tool in lipid biology research.
-
What is the full form of BODIPY in chemistry?
The full form of BODIPY in chemistry is Boron-dipyrromethene. These compounds consist of a boron atom coordinated with a dipyrromethene structure, exhibiting unique optical properties. With a compact structure and uniform electron distribution, BODIPY molecules display high fluorescence quantum yield, narrow emission spectra, and excellent photostability. Their structures are easily modifiable, allowing researchers to tune spectral characteristics by introducing substituents, thereby expanding their applications in fluorescence imaging, photosensitization, and molecular probe design.
-
What is the principle of BODIPY C11?
BODIPY C11 is a specialized fluorescent probe designed to detect lipid peroxidation. Its principle is based on structural changes during lipid peroxidation, including double bond cleavage and rearrangement, which result in significant shifts in fluorescence spectra. In its non-oxidized state, BODIPY C11 emits red fluorescence, whereas upon lipid peroxidation it shifts to green fluorescence. By monitoring the red-to-green fluorescence ratio, researchers can directly evaluate oxidative stress levels and lipid peroxidation within cells. This principle makes BODIPY C11 a highly valuable tool in studies of cellular damage and disease mechanisms.
More About BODIPY Dyes
Fluorescent Services

Fluorescent Beads
Bright, uniform microspheres for calibration and tracing.

Fluorescent Nanoparticles
Stable, bright nanoscale probes for imaging and sensing.
Fluorescent Proteins
Genetically encoded fluorescent markers for live-cell imaging.
Resources

- Hoechst Dyes: Definition, Structure, Mechanism and Applications
- Mastering the Spectrum: A Comprehensive Guide to Cy3 and Cy5 Dyes
- Fluorescent Probes: Definition, Structure, Types and Application
- Fluorescent Dyes: Definition, Mechanism, Types and Application
- Coumarin Dyes: Definition, Structure, Benefits, Synthesis and Uses
- Unlocking the Power of Fluorescence Imaging: A Comprehensive Guide
- Cell Imaging: Definitions, Systems, Protocols, Dyes, and Applications
- Lipid Staining: Definition, Principles, Methods, Dyes, and Uses
- Flow Cytometry: Definition, Principles, Protocols, Dyes, and Uses
- Nucleic Acid Staining: Definition, Principles, Dyes, Procedures, and Uses
Online Inquiry












