Green Fluorescent Protein: A Comprehensive Overview from Discovery to Application
Green fluorescent protein (GFP), a naturally fluorescent protein from jellyfish, has been used for years to allow real-time, visual tracking of proteins and cellular imaging without interfering with the normal functions of a cell. The structure of GFP has undergone over a decade of development and optimization, leading to the creation and refinement of numerous mutants with significantly enhanced brightness, photostability, and spectral properties. GFPs and their derivatives are now used across molecular biology, cell biology, neuroscience, and drug discovery in areas such as the study of gene expression, protein localization, and cellular dynamics, and bioimaging.
What is Green Fluorescent Protein?
GFP is a protein composed of 238 amino acids with a molecular weight of approximately 27 kilodaltons. Its core characteristic is its ability to absorb blue light (with two excitation peaks around 395 nm and 475 nm) and emit green light (around 508 nm). This makes GFP a highly valuable biological marker tool. The spontaneous fluorescence of GFP originates from an internal conjugated chromophore that forms during protein folding, creating a stable fluorescent center.
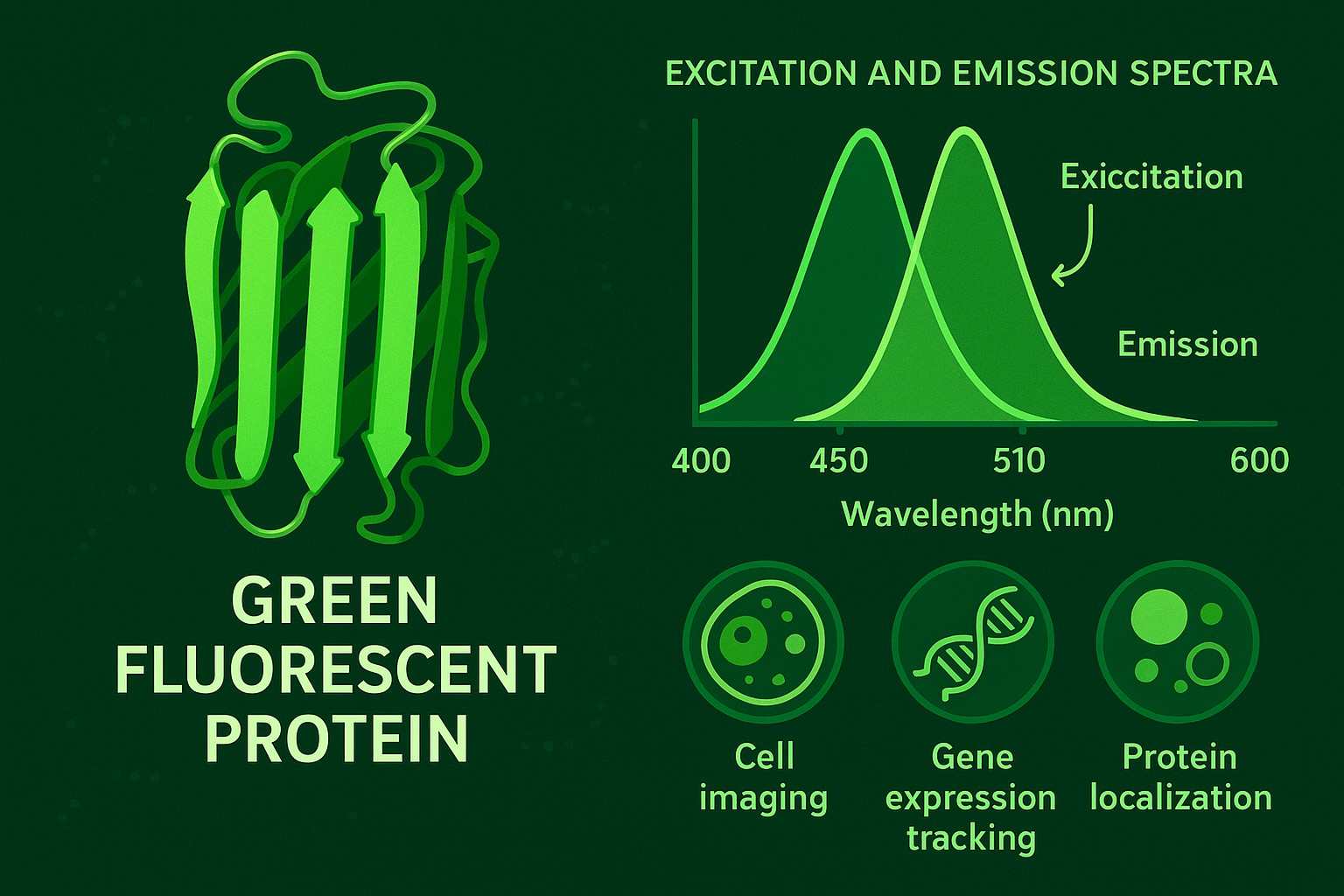 Fig. 1. Green fluorescent protein (BOC Sciences Authorized).
Fig. 1. Green fluorescent protein (BOC Sciences Authorized).
Where Does Green Fluorescent Protein Come From?
Green fluorescent protein was first discovered in the jellyfish Aequorea victoria, a bioluminescent jellyfish living along the Pacific Northwest coast. In the 1960s, Japanese scientist Osamu Shimomura first extracted and identified GFP from this jellyfish. The jellyfish's luminescence results from the synergistic effect of a photoprotein called aequorin and GFP. Specifically, aequorin emits blue light in the presence of calcium ions, and GFP absorbs this blue light and converts it into green light, producing the green glow we observe.
Subsequently, scientists successfully cloned the GFP gene, enabling its expression in various biological systems. This breakthrough greatly advanced the application of GFP in life science research. The characteristic of GFP being isolated from a natural organism and fluorescing without additional cofactors made it an ideal marker protein, ushering in a new era for gene reporting and protein localization studies in molecular and cellular biology.
Green Fluorescent Protein Structure
The three-dimensional structure of GFP presents a typical beta-barrel conformation, composed of 11 parallel beta-sheets that form a cylindrical structure around the center. Inside the internal cavity of this beta-barrel structure lies an autocatalytically formed chromophore, created from three specific amino acid residues within the protein chain (usually Ser65, Tyr66, and Gly67) during protein folding. The beta-barrel structure not only protects the chromophore from external chemical and physical influences but also provides a favorable microenvironment for the chromophore's stable fluorescence. The structural stability is a key reason why GFP maintains its function across different biological systems. Moreover, the overall conformation of GFP significantly impacts its optical properties and refractive index, allowing efficient absorption and emission of fluorescence.
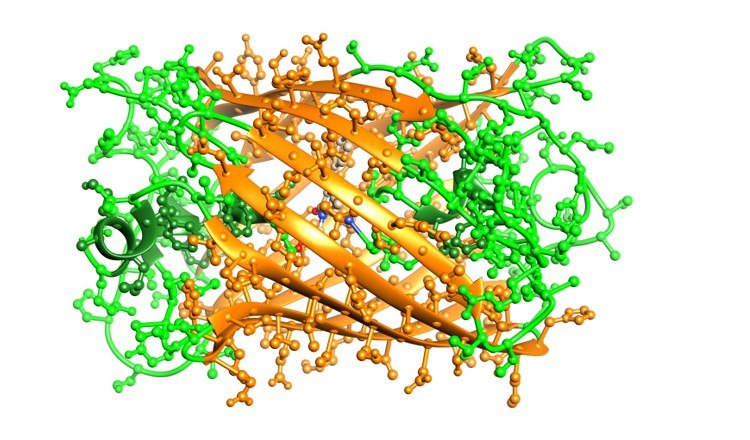 Fig. 2. Structure of green fluorescent protein (BOC Sciences Authorized).
Fig. 2. Structure of green fluorescent protein (BOC Sciences Authorized).
Green Fluorescent Protein Properties
In addition to GFP, many other fluorescent proteins of different colors have been discovered and developed, such as red fluorescent protein (RFP), blue fluorescent protein (BFP), yellow fluorescent protein (YFP), and others. Compared with these, GFP possesses several excellent physical and chemical properties:
- Optical characteristics: GFP exhibits two excitation peaks at 395 nm and 475 nm, with an emission peak at 508 nm, emitting bright green fluorescence.
- Spontaneous chromophore formation: The formation of GFP's chromophore depends entirely on the amino acid sequence and folding of the protein itself, without requiring any added coenzymes or cofactors, making it extremely convenient for live-cell and in vivo imaging.
- High stability: GFP has good tolerance to pH and temperature variations, suitable for use in different experimental environments. Its beta-barrel structure ensures the protein remains stably folded under various conditions.
- Low toxicity and biocompatibility: Expression of GFP in cells and organisms generally does not cause noticeable toxicity, making it suitable for long-term tracking studies.
- Fluorescence intensity and photostability: GFP has moderate fluorescence intensity and good photostability, resisting a certain degree of photobleaching, suitable for microscopy imaging and real-time monitoring.
Support Applications of Fluorescent Proteins from BOC Sciences
| Solutions | Description |
|---|---|
| Flow Cytometry | Provide high-quality fluorescent proteins optimized for flow cytometry to enable precise cell population analysis and sorting based on fluorescence intensity. |
| High-Throughput Screening | Support drug discovery and screening workflows with stable and bright fluorescent proteins compatible with automated high-throughput platforms for rapid and reliable readouts. |
| Drug Delivery | Offer fluorescent protein conjugates to track and monitor drug delivery systems in real time, improving understanding of biodistribution and targeting efficiency. |
| Cell Imaging | Supply diverse fluorescent protein variants with various spectral properties for detailed live-cell and fixed-cell imaging in fluorescence microscopy applications. |
| In Vivo Imaging | Provide near-infrared and other optimized fluorescent proteins suitable for deep-tissue imaging in live animal models, enhancing visualization of biological processes in vivo. |
Enhanced Green Fluorescent Protein and Other Variants
With the development of molecular biology and cell imaging technologies, scientists have continuously engineered GFP to optimize its performance for more complex experimental needs. Enhanced green fluorescent protein (EGFP) and various other GFP variants have been developed, greatly enriching the chromatic and functional options for fluorescent labeling. These modified proteins not only improve fluorescence brightness and stability but also expand the range of applications, providing powerful tools for multicolor imaging, dynamic monitoring, and biological labeling in special environments.
Enhanced Green Fluorescent Protein
Enhanced green fluorescent protein is a highly efficient variant derived from wild-type GFP through genetic engineering. EGFP introduces key mutations (such as F64L and S65T) in the wild-type GFP amino acid sequence, significantly enhancing its fluorescence intensity, folding efficiency, and expression levels. These improvements have made EGFP one of the most widely used fluorescent proteins in molecular biology and cell imaging research today. Compared with the original GFP, EGFP offers the following advantages:
- Stronger fluorescence brightness, providing clearer and easier-to-detect signals;
- Excitation wavelength closer to 488 nm, compatible with commonly used laser confocal microscopes;
- Better thermal and photostability, suitable for prolonged imaging;
- Faster maturation speed, improving real-time monitoring capabilities.
Other Variants of Green Fluorescent Protein
Beyond EGFP, researchers have developed multiple GFP-derived variants to meet diverse experimental requirements. These diverse variants have enriched the fluorescent protein palette and application scope, allowing researchers to flexibly choose appropriate markers according to experimental designs.
- Blue Fluorescent Protein (BFP): Derived from GFP through specific amino acid mutations, emits blue fluorescence, suitable for multicolor imaging.
- Yellow Fluorescent Protein (YFP): Engineered from GFP to emit yellow fluorescence, commonly used in colocalization and FRET experiments.
- Superfolder GFP (sfGFP): Exhibits higher folding efficiency and stability, suitable for complex cellular environments.
- Photoswitchable GFP: Fluorescence state can be altered by light exposure, enabling dynamic control within live cells.
How Does Green Fluorescent Protein Work?
Green fluorescent protein is able to emit bright green fluorescence mainly due to its unique internal chromophore structure and its photophysical properties. The GFP chromophore is formed through an autocatalytic reaction involving three amino acid residues within the protein itself (Ser65, Tyr66, and Gly67) after the protein folds correctly, without requiring any external cofactors or enzymatic reactions. This spontaneously formed chromophore endows GFP with its unique ability to fluoresce spontaneously. Its working mechanism can be divided into the following steps:
- Excitation light absorption: When GFP is exposed to light of specific wavelengths (mainly ultraviolet or blue light at approximately 395 nm and 475 nm), the chromophore absorbs photon energy, causing electrons to transition to an excited state.
- Energy conversion and emission: The excited electrons return to the ground state in a very short time, releasing energy in the form of green light at a longer wavelength (around 508 nm), which is the green fluorescence we observe.
- Protective role of the β-barrel structure: The β-barrel structure of GFP not only ensures stable protein folding but also protects the chromophore from environmental interference (such as water molecules or chemical reagents), thereby enhancing fluorescence stability and efficiency.
- No need for cofactors: GFP can autonomously fold and form the chromophore inside live cells without adding any external chemicals or auxiliary enzymes, making it an ideal tool for live-cell labeling and imaging.
- Prokaryotic expression systems: such as E. coli, suitable for rapid expression and purification of GFP protein, facilitating structural and functional studies.
- Eukaryotic expression systems: such as yeast, mammalian cells, and plant cells, suitable for studying protein function and localization in complex cellular environments.
Diverse Fluorescent Protein Product Supply Capability
- Wide range of fluorescent protein products, including GFP, RFP, and multiple spectral variants.
- Products cover different emission wavelengths to meet multi-channel imaging needs.
- High product purity, stable batch-to-batch consistency, and various specifications available.
- Stable inventory and flexible supply schedules to support bulk purchasing.
Professional Fluorescent Protein Custom Synthesis Capability
- Gene sequence design and optimization to meet personalized requirements.
- Support for multiple expression systems: E. coli, yeast, mammalian cells, etc.
- Development of efficient protein expression and purification processes.
- Structural modification and functional optimization of fluorescent proteins.
Fluorescent Protein Labeling and Conjugation Services
- Multiple labeling and conjugation techniques covering covalent and non-covalent bindings.
- Efficient conjugation of fluorescent proteins with antibodies, small molecule ligands, nanoparticles, and other carriers.
- Ensuring excellent fluorescence performance and biological activity of labeled proteins.
- Applications in cell imaging, flow cytometry, biosensing, targeted drug delivery, and molecular diagnostics.
Applications of Green Fluorescent Protein
Due to its unique spontaneous fluorescence property and genetic manipulability, GFP has become an indispensable tool in life science research. Its applications span cell biology, molecular biology, developmental biology, and disease research, providing scientists with an intuitive, dynamic, and non-invasive observation method.
Application of Green Fluorescent Protein in Cell Biology
In cell biology, GFP is widely used to study the localization, dynamic changes, and interactions of proteins inside cells. By fusing the GFP gene to the target protein gene, researchers can track the distribution of proteins in real time and monitor dynamic changes in organelles such as mitochondrial movement, cytoskeleton remodeling, and transport within the endoplasmic reticulum and Golgi apparatus. Additionally, GFP technology is used to observe real-time changes in the cell cycle, cell division, apoptosis, and signaling pathways, greatly advancing the understanding of cellular functions and behaviors.
Application of Green Fluorescent Protein in Molecular Biology
GFP plays an important role as a reporter gene in molecular biology. It is commonly used for promoter activity analysis, gene expression regulation studies, and transcription factor function verification. By measuring GFP fluorescence intensity, researchers can quantitatively assess gene expression changes under different conditions. Furthermore, GFP is used as a tool for studying protein interactions; using Förster Resonance Energy Transfer (FRET) technology, it reveals close contacts and interactions between molecules, providing strong support for molecular mechanism analysis.
Application of Green Fluorescent Protein in Developmental Biology
In developmental biology research, the application of GFP has greatly enriched the understanding of cell fate and tissue formation processes. Scientists generate transgenic animals expressing GFP specifically in certain cell types or developmental stages to achieve dynamic tracking of cell migration, differentiation, and tissue remodeling. This technique not only reveals the spatiotemporal relationships of embryonic development but also helps study gene regulatory networks and morphogenesis mechanisms in multicellular development.
Application of Green Fluorescent Protein in Disease Research and Diagnosis
GFP also plays an important role in disease research and clinical diagnosis. By labeling tumor cells or pathogens with GFP, researchers can observe their growth, spread, and interactions with host cells in vivo, thereby gaining deeper insight into disease progression. Meanwhile, GFP technology supports high-throughput drug screening and target validation, accelerating new drug development. In some clinical diagnostic studies, GFP is also used as a biological marker to assist in detecting specific disease-related molecules, promoting the advancement of precision medicine.
Green Fluorescent Protein Laboratory Techniques
Green fluorescent protein is not only widely used as a research tool, but its laboratory techniques have also become increasingly mature and diverse. The following introduces GFP gene cloning and expression, protein purification and detection, and commonly used GFP tagging techniques to help researchers efficiently utilize this powerful tool.
Green Fluorescent Protein Gene
The GFP gene was first cloned from the jellyfish Aequorea victoria, with a length of about 720 base pairs encoding a 238-amino-acid protein. The successful cloning of this gene marked the transition of GFP from a natural product to a biotechnological tool and laid the foundation for its heterologous expression in various biological systems.
The GFP gene has a highly conserved coding sequence, especially in the critical amino acid regions responsible for chromophore formation. Through genetic engineering, researchers have optimized and modified the GFP gene in various ways, such as codon optimization and introduction of mutations, to enhance its expression efficiency and fluorescent properties. These modifications make the GFP gene more suitable for expression systems in different host cells, including bacteria, yeast, plants, and mammalian cells. In addition, the GFP gene is usually cloned into expression vectors containing strong promoters and regulatory elements to achieve efficient and stable gene expression and protein yield.
Expression of Green Fluorescent Protein
GFP expression technology is the foundation for its widespread application. Through molecular cloning, the GFP gene can be fused with target genes to form fusion proteins, allowing real-time localization and dynamic observation of target proteins. Regarding expression systems, GFP can be effectively expressed in multiple hosts:
GFP gene expression is usually achieved via transfection or transgenic technology. The expressed product can be directly observed under a fluorescence microscope, eliminating traditional staining steps and greatly facilitating live-cell and in vivo imaging. Additionally, the expressed GFP protein correctly folds intracellularly and autonomously forms the chromophore, a process independent of external enzymes or cofactors, making GFP a powerful tool for live-cell biology research.
Purification and Detection of Green Fluorescent Protein
GFP purification commonly uses a combination of affinity chromatography, ion-exchange chromatography, and gel filtration to obtain high-purity protein. Fusion tags (such as His-tags) assist purification and significantly improve efficiency. For detection, fluorescence microscopy and fluorescence spectrophotometry are primary tools for observing and measuring GFP fluorescence signals. SDS-PAGE combined with fluorescence scanning is also widely used for qualitative analysis of protein expression and purity. Moreover, flow cytometry combined with fluorescence detection enables high-throughput quantitative analysis of GFP expression in cell populations.
Green Fluorescent Protein Tagging Techniques
GFP tagging involves fusing the GFP gene with the target protein gene, expressing fusion proteins to localize and dynamically monitor target proteins. Common fusion approaches include N-terminal and C-terminal fusions, chosen based on the functional domains and spatial conformation of the target protein. Besides genetic fusion, immunofluorescence staining combined with GFP is also used to verify expression and localization. In recent years, developments in photo-switchable GFP and split-GFP technologies have provided more flexible tools for studying protein interactions and cell signaling.
Fluorescent Protein Services Offered by BOC Sciences
BOC Sciences is dedicated to providing high-quality fluorescent protein and its derivatives for life science research, meeting diverse scientific needs. Whether for basic research or cutting-edge applications, we offer comprehensive solutions to help researchers achieve outstanding results.
Do You Need A Consultation?
BOC Sciences integrates cutting-edge fluorescence technologies to accelerate your research, driving next-generation solutions for drug discovery and diagnostics.
Transform Your Studies with Cutting-Edge Fluorescent Products
| Cat. No. | Product Name | CAS No. | Inquiry |
|---|---|---|---|
| F01-0074 | 2,6-Diiodo-1,3,5,7,8-pentaethyl-BODIPY | 1031443-55-6 | Inquiry |
| R01-0009 | BDY FL, SE | 146616-66-2 | Inquiry |
| F01-0166 | BODIPY 493/503 NHS Ester | 216961-98-7 | Inquiry |
| A16-0033 | 6-Carboxyfluorescein | 3301-79-9 | Inquiry |
| A16-0170 | Rhodamine-123 | 62669-70-9 | Inquiry |
| A19-0040 | Hoechst 33342 | 23491-52-3 | Inquiry |
| A16-0201 | DAPI dihydrochloride | 28718-90-3 | Inquiry |
| A14-0036 | Rhodamine B hydrazide | 74317-53-6 | Inquiry |
| F06-0011 | Coumarin 153 | 53518-18-6 | Inquiry |
| F02-0026 | Cy5-NHS ester | 146368-14-1 | Inquiry |
High-Performance Fluorescent Tools for Your Research
- Click Chemistry Reagents Enable efficient and bioorthogonal labeling for diverse applications.
- Fluorescent Dyes Bright, stable dyes for imaging and detection needs.
- Fluorescent Probes Specific probes for monitoring biological targets and processes.
- Fluorescent Nanoparticles Advanced particles for enhanced imaging and sensing performance.
More About Fluorescent Proteins
Online Inquiry

