Fluorescent Proteins in Bioimaging: Challenges and Innovative Solutions
Fluorescent proteins, as core tools in bioimaging technology, leverage their unique intrinsic fluorescence and genetic encoding advantages to enable highly specific labeling and dynamic tracking of molecules and structures within living cells. In addressing challenges such as photobleaching, signal attenuation, and nonspecific labeling during bioimaging, fluorescent proteins offer researchers powerful solutions due to their excellent biocompatibility, customizability, and diverse spectral properties.
Introduction to Fluorescent Proteins in Bioimaging
As vital labeling tools in the field of bioimaging, fluorescent proteins are widely applied in cell tracking, protein localization, and dynamic process observation because of their unique intrinsic fluorescence and high specificity. Benefiting from their genetic encoding, fluorescent proteins can be stably expressed in living cells, providing robust visualization capabilities that have propelled advancements in molecular and cellular biology.
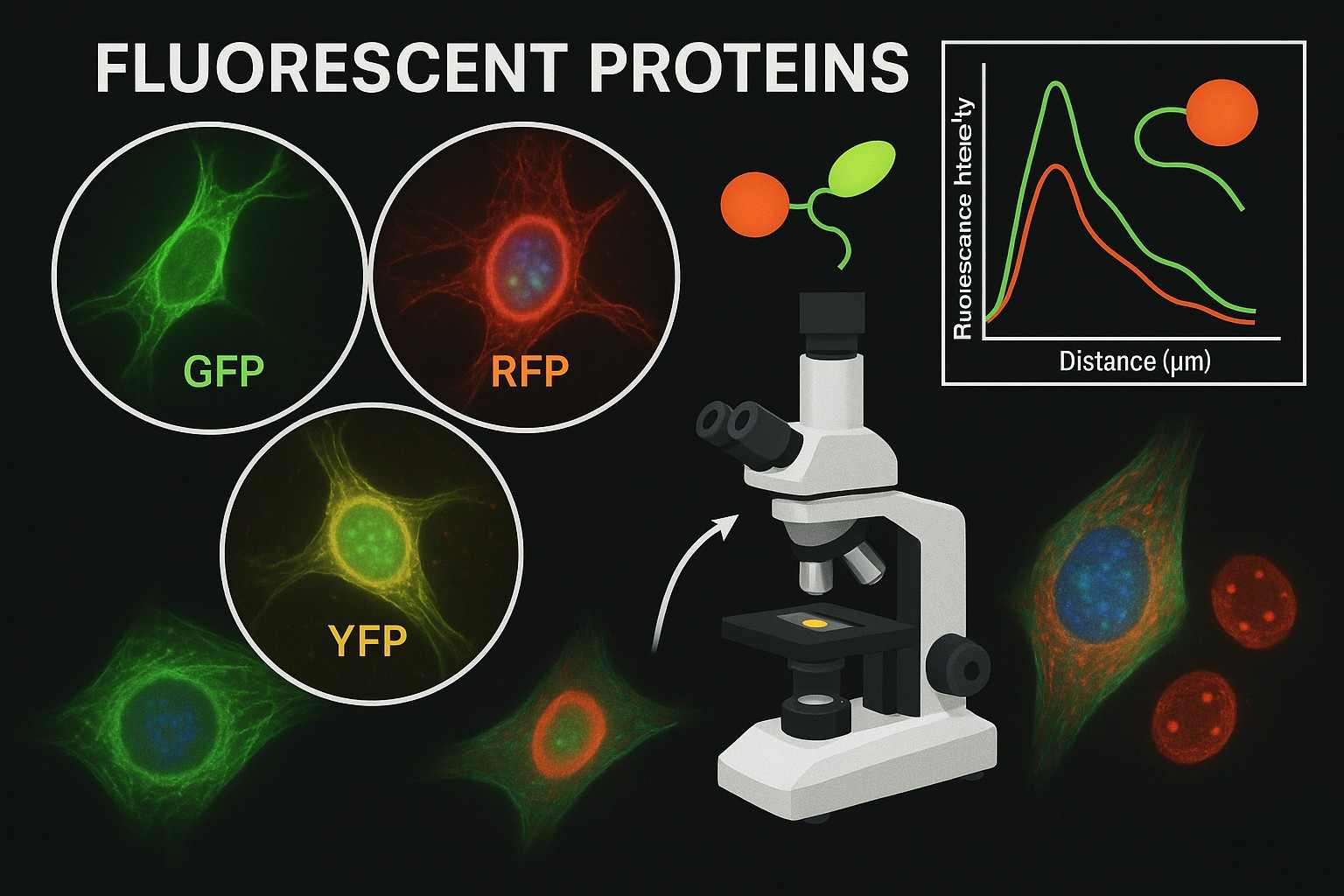 Fig. 1. Fluorescent proteins (BOC Sciences Authorized).
Fig. 1. Fluorescent proteins (BOC Sciences Authorized).
What Are Fluorescent Proteins?
Fluorescent proteins are a class of natural proteins that absorb excitation light at specific wavelengths and emit fluorescence at longer wavelengths. The first to be discovered and widely applied was green fluorescent protein (GFP) from the jellyfish Aequorea victoria. Its unique chromophore structure enables fluorescent proteins to emit intense and stable fluorescence signals at the molecular level. Through genetic engineering, fluorescent proteins can serve as molecular markers introduced into various cells and organisms, enabling real-time observation and tracking of cellular structures, molecular dynamics, and biological processes. This genetically encoded property makes fluorescent proteins invaluable tools in bioimaging.
Key Types of Fluorescent Proteins Used in Imaging
Based on differences in excitation and emission spectra, fluorescent proteins are classified into various types to meet diverse imaging needs. These proteins are continually engineered to improve brightness, photostability, spectral diversity, and environmental adaptability, addressing complex requirements in research:
- Green Fluorescent Proteins (GFP) and variants: such as EGFP, offering high brightness and good photostability, making them the most commonly used fluorescent proteins.
- Red Fluorescent Proteins (RFP) and derivatives: such as mCherry and DsRed, with longer emission wavelengths that help reduce tissue autofluorescence interference, suitable for deep-tissue imaging.
- Yellow Fluorescent Proteins (YFP): with spectra between green and red, ideal for multicolor imaging and energy transfer experiments.
- Blue Fluorescent Proteins (BFP) and Cyan Fluorescent Proteins (CFP): used in specialized multicolor labeling and detection of molecular interactions.
- Near-Infrared Fluorescent Proteins (NIR FP): emitting in the near-infrared range to enable non-invasive imaging of deeper tissues.
Advantages of Fluorescent Proteins for Imaging Applications
Compared with traditional fluorescent dyes or fluorescent probes, fluorescent proteins have significant advantages. These benefits make them irreplaceable molecular imaging tools in life sciences, driving breakthroughs in cell biology, developmental biology, neuroscience, and beyond:
- Genetic encoding expression: enables direct expression in target cells or organisms via gene transfection, supporting dynamic live imaging.
- Low cytotoxicity: suitable for long-term observation with minimal interference to cellular physiology.
- Multicolor spectral selection: supports multi-target, complex multicolor imaging experiments.
- High-specificity localization: allows fusion with target proteins for precise subcellular localization and dynamic tracking.
- Engineering flexibility: facilitates customization for specific experimental needs, such as enhanced brightness, photostability, or environmental sensitivity.
Common Challenges in Bioimaging Research
Bioimaging, as a critical tool in modern life sciences, is widely applied in cell observation, molecular tracking, and disease research. However, as studies advance, researchers continue to face numerous challenges related to imaging resolution, signal stability, specific labeling, and biocompatibility.
Limited Photostability of Fluorescent Probes
Fluorescent probes are prone to photobleaching under excitation light, causing fluorescence signals to weaken or disappear, which limits applications in long-term and repeated imaging. Especially under high-intensity light sources or prolonged exposure, the probe's structure may degrade, compromising image quality and data continuity. Photobleaching not only reduces image brightness but also increases errors in quantitative analysis. Enhancing probe photostability is thus key to achieving high-quality dynamic bioimaging.
Spectral Overlap in Multicolor Imaging
Multicolor imaging relies on different fluorescent probes emitting at distinct wavelengths, but overlap between excitation and emission spectra often leads to signal crosstalk, hampering accurate distinction between labels. Spectral overlap reduces imaging resolution and reliability, complicating data interpretation. Solving this issue requires developing fluorescent proteins with narrow, non-overlapping emission peaks or employing spectral separation and computational deconvolution techniques to improve clarity and accuracy in multi-channel imaging.
Biocompatibility and Toxicity in In Vivo Imaging
Fluorescent probes must exhibit good biocompatibility to avoid toxic effects on cells or tissues. Some chemical dyes and nanomaterials contain harmful components that may induce cellular stress or death, limiting their use in vivo. The immunogenicity and metabolic stability of probes also affect imaging outcomes and safety. Selecting low-toxicity, non-immunogenic, and metabolically friendly probes is fundamental to achieving long-term, non-invasive in vivo imaging.
Insufficient Targeting and Specificity
High targeting ability and specificity are crucial for accurate imaging, as nonspecific binding increases background signals and obscures true targets. In complex biological environments, nonspecific binding can generate false positives, undermining result reliability. Strategies such as using specific antibodies or protein fusion tags can improve probe binding precision. Optimizing probe affinity and minimizing nonspecific interactions help enhance imaging accuracy and credibility.
Support Applications of Fluorescent Proteins from BOC Sciences
| Solutions | Description |
|---|---|
| Molecular Imaging | Facilitate high-resolution molecular imaging to study complex biological interactions in real time. |
| High-Throughput Screening | Support large-scale compound screening with fluorescent proteins as reliable and sensitive reporters. |
| Cell Imaging | Enable detailed visualization of cellular structures, dynamics, and pathways for advanced research. |
| In Vivo Imaging | Advance in vivo studies with fluorescent proteins for non-invasive monitoring of biological processes in living systems. |
How Fluorescent Proteins Address Bioimaging Bottlenecks?
With advances in protein engineering and molecular biology, scientists have successfully designed and optimized the optical properties and biocompatibility of fluorescent proteins, leading to the development of a series of novel high-performance fluorescent proteins. These innovative proteins significantly enhance imaging brightness and stability while adapting to complex cellular and tissue environments, greatly overcoming traditional fluorescence imaging bottlenecks and offering researchers more powerful and flexible tools.
Providing High-Specificity Labeling
Fluorescent proteins can be fused directly with target proteins via genetic engineering, achieving high-specificity labeling. Unlike traditional dye labeling, this method avoids nonspecific binding issues and precisely localizes specific proteins or structures within cells. For example, GFP can be fused to cytoskeletal proteins, allowing researchers to clearly observe dynamic changes in the cytoskeleton within living cells. Additionally, genetically encoded fluorescent protein tags ensure continuous and stable labeling, suitable for long-term tracking studies.
Improving Biocompatibility
As endogenous protein expression products, fluorescent proteins naturally exhibit excellent biocompatibility and low toxicity, making them ideal for live-cell and in vivo imaging. Compared to chemically synthesized dyes and nanoparticle probes, fluorescent proteins cause minimal disruption to cellular function. For instance, in neuroscience research, fluorescent protein labeling of specific neurons enables non-invasive long-term observation, avoiding stress responses that dyes may cause and ensuring data authenticity and reliability.
Enabling Multicolor Imaging
Different fluorescent proteins with distinct excitation and emission spectra—such as GFP, RFP, and YFP—support multi-target and multi-channel simultaneous imaging. By combining them appropriately, multiple markers can be visualized within the same cell or tissue, revealing complex molecular interactions and spatial distributions. For example, in tumor microenvironment studies, different fluorescent proteins can label tumor cells, immune cells, and blood vessels to simultaneously monitor their interactions and dynamic changes.
Customizable and Optimizable Properties
Through protein engineering, the photostability, brightness, and spectral characteristics of fluorescent proteins can be finely tuned to meet various experimental needs. For instance, superfolder GFP (sfGFP) has been modified to possess stronger photostability and higher folding efficiency, making it suitable for use in harsh or complex cellular environments. Furthermore, scientists have developed red, near-infrared, and other fluorescent proteins, expanding the spectral range for imaging and enhancing the ability to image deep tissues.
Applications of Fluorescent Proteins in Bioimaging
The diverse optical properties and genetic encoding characteristics of fluorescent proteins make them widely applied in dynamic observation of cells and organisms, molecular process tracking, and functional detection. Whether studying molecular mechanisms within cells or performing non-invasive imaging throughout entire organisms, fluorescent proteins have greatly advanced the frontiers of life sciences.
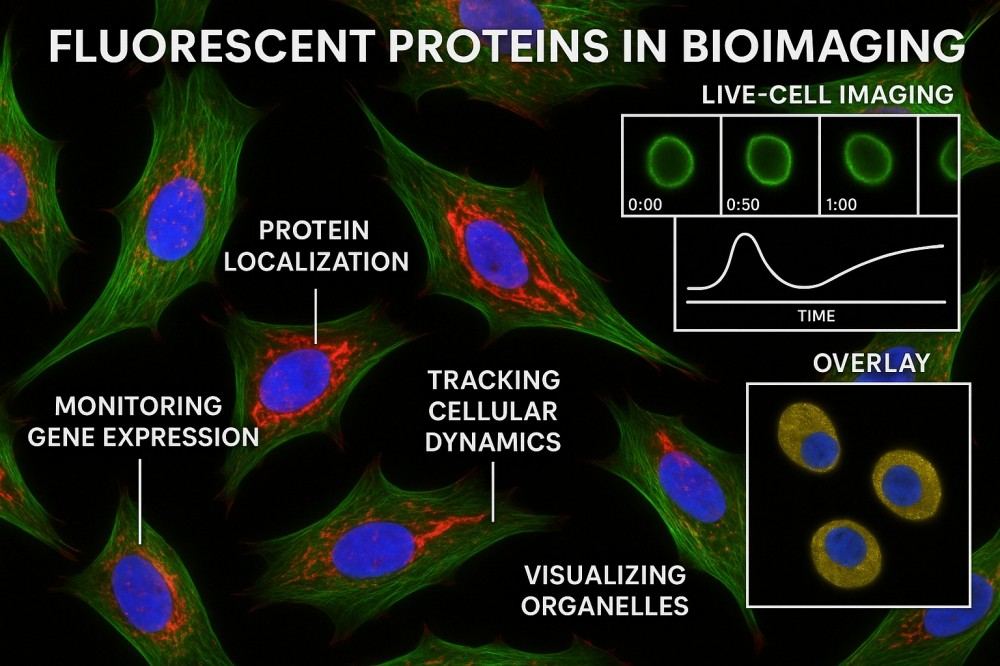 Fig. 2. Fluorescent proteins in bioimaging (BOC Sciences Authorized).
Fig. 2. Fluorescent proteins in bioimaging (BOC Sciences Authorized).
Live-Cell Imaging for Dynamic Cellular Processes
Fluorescent proteins enable real-time labeling of various molecular structures and dynamic processes within living cells, including cell division, migration, and signal transduction. Through gene fusion expression, fluorescent proteins precisely localize target proteins, providing visual information on complex intracellular dynamics. Live-cell imaging not only observes individual cell behavior but also reveals intercellular interactions, helping scientists understand cellular functions and their changes under physiological and pathological conditions.
Tracking Gene Expression and Protein Localization
As reporter genes, fluorescent proteins can reflect the spatiotemporal distribution of gene expression. By monitoring changes in fluorescence intensity in real time, researchers can accurately evaluate gene activity under different conditions or developmental stages. Simultaneously, fluorescent protein-labeled protein localization reveals their precise distribution within cells, aiding in deciphering protein functions and organelle dynamics. This approach is suitable not only for basic studies on gene regulation but also for disease mechanism and drug action research, enhancing the visualization capabilities of molecular biology experiments.
In Vivo Imaging for Whole-Organism Studies
With the use of near-infrared and red-shifted fluorescent proteins, non-invasive in vivo imaging techniques have become possible. Fluorescent proteins are utilized in transgenic animal models to support comprehensive observation of disease progression, organ function, and drug metabolism. This imaging method reduces harm to animals and enables dynamic, continuous monitoring. In vivo imaging has greatly propelled research in oncology, neuroscience, and developmental biology, providing more authentic biological information than traditional tissue sectioning.
Fluorescent Biosensors for Detecting Cellular Signals
Biosensors based on fluorescent proteins can sensitively detect physiological indicators within cells, such as calcium ion concentration, pH value, and redox states. The environment-sensitive properties of fluorescent proteins allow them to emit quantifiable signals upon specific stimuli, achieving dynamic monitoring of cellular functional states. These sensors play an important role in studying cellular metabolism, signaling pathways, and environmental responses, providing powerful tools for understanding cellular regulation mechanisms and disease states.
Visualization of Protein-Protein Interactions
Fluorescent proteins offer unique advantages in elucidating protein-protein interactions. Using FRET technology, researchers can detect whether two fluorescent protein-labeled proteins are in close proximity, revealing the dynamics of protein interactions. BiFC technology visualizes complex formation through molecular complementation. These techniques enable scientists to deeply understand complex intracellular signaling and regulatory networks, offering key insights for exploring disease mechanisms and target screening.
High-Throughput Screening Applications in Drug Discovery
Fluorescent protein-labeled cells and molecular systems are widely employed in drug screening and target validation. High-throughput imaging platforms combined with the high sensitivity of fluorescent proteins make it possible to rapidly screen thousands of compounds. Researchers can observe, in real time, the effects of drugs on cell morphology, function, and molecular expression, significantly improving drug discovery efficiency. Such applications not only reduce research and development costs but also accelerate the transition of new drugs from the laboratory to clinical application.
Fluorescent Protein Services Designed to Empower Your Research
To help researchers overcome technical challenges in bioimaging, BOC Sciences provides comprehensive fluorescent protein products and services. We offer diverse fluorescent protein variants, advanced protein engineering technologies, and professional conjugation and labeling solutions, committed to delivering high-quality, customized research support to enable precise and efficient imaging and analysis in life sciences.
Supply of Diverse Fluorescent Protein Variants
- Provide classic and innovative fluorescent proteins, including GFP, RFP, YFP, CFP, and near-infrared fluorescent proteins.
- Broad spectral coverage to meet multicolor imaging and complex sample needs.
- High purity and excellent optical performance ensure reliable experimental data.
- Suitable for live-cell imaging, in vivo imaging, and various biological samples.
Custom Engineering and Optimization Services
- Gene sequence design and site-directed mutagenesis for protein performance optimization.
- Enhanced fluorescence brightness and photostability.
- Optimized environmental adaptability, such as pH sensitivity and red-shift characteristics.
- Personalized customization services to meet specific research requirements.
Conjugation and Labeling Solutions for Bioimaging Applications
- Efficient conjugation of fluorescent proteins with antibodies, small molecules, or nanomaterials.
- Optimized chemical crosslinking strategies to ensure labeling stability and activity.
- Diverse labeling solutions supporting both intracellular and extracellular imaging applications.
- Provision of tailor-made labeling reagents to meet different experimental design needs.
Do You Need A Consultation?
BOC Sciences integrates cutting-edge fluorescence technologies to accelerate your research, driving next-generation solutions for drug discovery and diagnostics.
Cutting-Edge Fluorescent Tags for Proteins
| Cat. No. | Product Name | CAS No. | Inquiry |
|---|---|---|---|
| A01-0019 | Fluorescein-dT Phosphoramidite | N/A | Inquiry |
| A01-0018 | 6-Fluorescein Serinol Phosphoramidite | 1275574-87-2 | Inquiry |
| A01-0020 | Yakima Yellow® Phosphoramidite | 502485-39-4 | Inquiry |
| R01-0472 | Atto 425-NHS ester | 892156-28-4 | Inquiry |
| A01-0011 | MSBN | 135980-66-4 | Inquiry |
| A01-0015 | 6-NED acid | 327174-92-5 | Inquiry |
| F04-0026 | FAM-xtra Phosphoramidite | 2304636-67-5 | Inquiry |
| F04-0027 | Fluorescein II CEP | 1027512-13-5 | Inquiry |
| A01-0013 | VIC phosphoramidite, 6-isomer | 1414265-81-8 | Inquiry |
| A01-0009 | Acridine Orange compd. with zinc chloride | 10127-02-3 | Inquiry |
High-Performance Fluorescent Tools for Your Research
- ICG Dyes Near-infrared dyes for deep tissue imaging and precise protein labeling.
- Alexa Fluor Bright, photostable dyes enabling highly sensitive protein detection.
- Cyanine Flexible multicolor dyes for advanced protein labeling applications.
- Fluorescent Nanoparticles High-sensitivity probes offering stable and robust protein labeling.
More About Fluorescent Proteins
Online Inquiry

