Phthalocyanine Dyes
Phthalocyanine is a compound with a large conjugated system of 18 electrons, and its structure is very similar to that of porphyrins widely found in nature. However, unlike porphyrins, which play an important role in living organisms, phthalocyanines Is a completely synthetic compound.
Structure
In summary, porphyrin molecules have the following characteristics: (1) has a special two-dimensional conjugated π-electron structure. (2) High stability to light and heat. (3) The molecular structure is diverse and easy to cut. Molecules can be derived from a variety of substituted ligands, and ligands can be designed, tailored, and assembled based on synthetic goals. (4) The coordination ability is very strong, it can coordinate with almost all metal elements in the periodic table to form complexes. Due to the above characteristics, there are many types of phthalocyanine compounds, each with its own characteristics and widely used. It is known that holes in the center of phthalocyanine can be matched with more than seventy metals. For transition metals, single-layer phthalocyanine complexes are generally formed, while rare earth metal phthalocyanines exist as sandwich complexes. The choice of the central metal greatly affects the physicochemical properties of the complex.
 Figure 1. Structure of Phthalocyanine.
Figure 1. Structure of Phthalocyanine.
Application
Phthalocyanines are a class of macrocyclic conjugate complexes of aromatic conjugated systems with 16 centers and 18π electrons composed of 8 N atoms and 8 C atoms. It has bright colors, low production cost, excellent colorability, good light, thermal and chemical stability, excellent light and electrical properties, good absorption in the visible light region, and adjustable molecular structure. In addition to being used as traditional dyes and pigments, phthalocyanine compounds have long been used as photosensitizers in solar cells. At the same time, there is a hole in the phthalocyanine ring, which can contain iron, copper, cobalt, aluminum, nickel, calcium, sodium, magnesium, zinc and other metal elements, and combine to form a metal complex. Metal phthalocyanine compounds with different energy levels can be obtained by changing different metal ions, which is beneficial to improve the photoelectric conversion efficiency of solar cells. However, the unsubstituted metal phthalocyanine is almost insoluble in water and organic solvents, which greatly limits its application. The method to improve the water solubility of metal phthalocyanines is generally to add a sulfonic or carboxylic acid group to the benzene ring.
Related compound
Phthalocyanines are structurally related to other tetrapyrrole macrocyles including porphyrins and porphyrazines. They feature four pyrrole-like subunits linked to form a 16-membered inner ring composed of alternating carbon and nitrogen atoms. Structurally larger analogues include naphthalocyanines. The pyrrole-like rings within H2Pc are closely related to isoindole. Both porphyrins and phthalocyanines function as planar tetradentate dianionic ligands that bind metals through four inwardly projecting nitrogen centers. Such complexes are formally derivatives of Pc2−, the conjugate base of H2Pc.
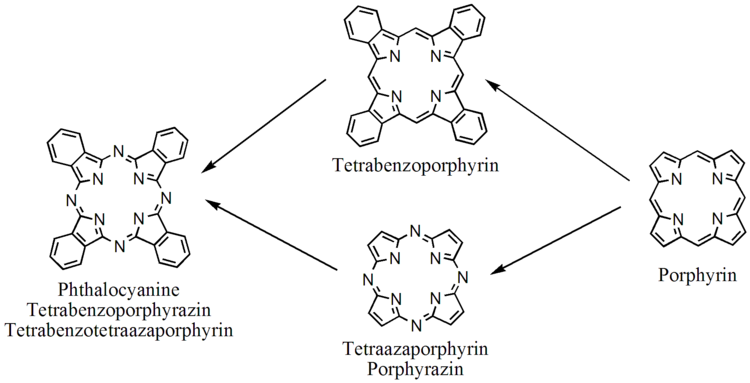 Figure 2. Relationship of the phthalocyanine with the porphyrin macrocycle. Two intramacrocyclic N-H groups are omitted.
Figure 2. Relationship of the phthalocyanine with the porphyrin macrocycle. Two intramacrocyclic N-H groups are omitted.
Reference:
- Iannuzzi, Marcella.; et al. Site-selective adsorption of phthalocyanine on h-BN/Rh (111) nanomesh. Physical Chemistry Chemical Physics. 2014, 16 (24): 12374–84.
Resources

- Hoechst Dyes: Definition, Structure, Mechanism and Applications
- Mastering the Spectrum: A Comprehensive Guide to Cy3 and Cy5 Dyes
- Fluorescent Probes: Definition, Structure, Types and Application
- Fluorescent Dyes: Definition, Mechanism, Types and Application
- Coumarin Dyes: Definition, Structure, Benefits, Synthesis and Uses
- Unlocking the Power of Fluorescence Imaging: A Comprehensive Guide
- Cell Imaging: Definitions, Systems, Protocols, Dyes, and Applications
- Lipid Staining: Definition, Principles, Methods, Dyes, and Uses
- Flow Cytometry: Definition, Principles, Protocols, Dyes, and Uses
- Nucleic Acid Staining: Definition, Principles, Dyes, Procedures, and Uses
Online Inquiry

