Glutathione (GSH) Probes
Glutathione (GSH) is an antioxidant in plants, animals, fungi, and certain bacteria and archaea. Glutathione prevents the destruction of important cellular components by reactive oxygen species such as free radicals, peroxides, lipid peroxides and heavy metals. It is a γ-peptide bond between the carboxyl group and the cysteine of the glutamic acid. Peptide. The carboxyl group of the cysteine residue is linked to the glycine by a normal peptide bond. Glutathione can help maintain normal immune system function, and has antioxidant effects, integrated detoxification. The thiol group on cysteine is its active group (so often abbreviated as G-SH), and it is easy to combine with certain drugs, toxins, etc., so that it has integrated detoxification effect. Glutathione can be used not only as a medicine, but also as a base for functional foods, and is widely used in functional foods such as anti-aging, immunity enhancement, and anti-tumor. Glutathione has both reduced (G-SH) and oxidized (G-S-S-G) forms, and most of the reduced glutathione under physiological conditions. Glutathione reductase can catalyze the mutual transformation between the two types. The enzyme's coenzyme can also provide NADPH for pentose phosphate bypass metabolism.
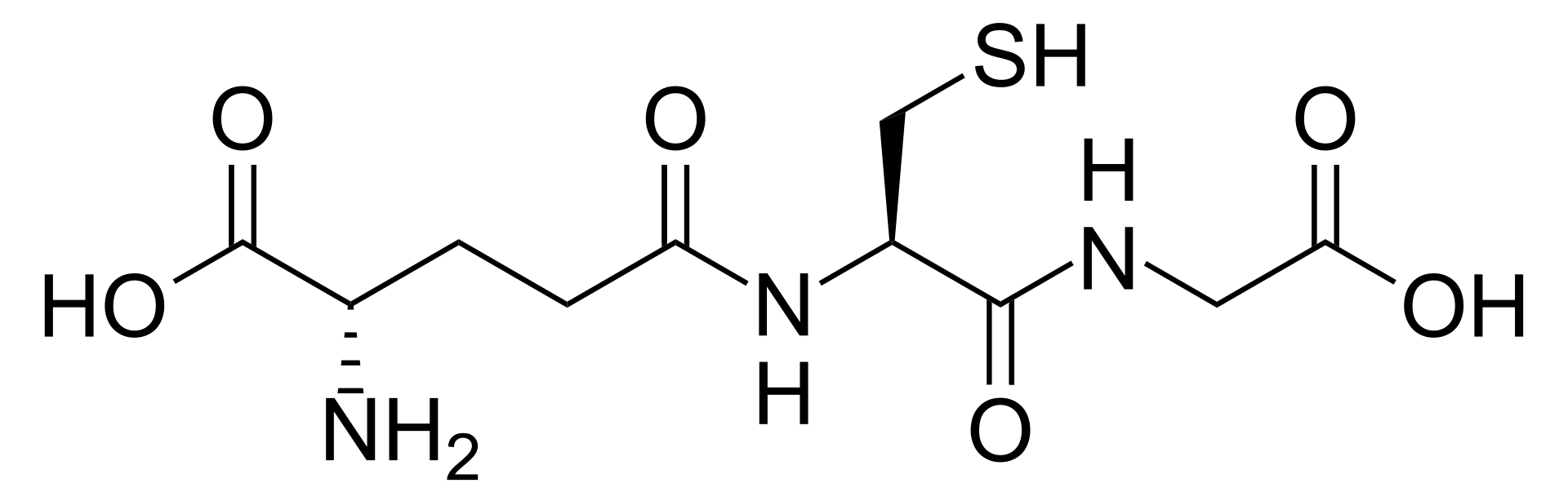 Figure 1. Chemical structure of Glutathione.
Figure 1. Chemical structure of Glutathione.
Mechanism
GSH is an important regulatory substance in the cell. It is a prosthetic group of glyceraldehyde phosphate dehydrogenase, a coenzyme of glyoxalase and triose dehydrogenase, and participates in the in vivo tricarboxylic acid cycle and glucose metabolism. It can activate a variety of enzymes, such as sulfhydryl (SH) enzymes - coenzymes, to promote the metabolism of sugars, fats and proteins. GSH is characterized by its active sulfhydryl group (-SH), which is the most important functional group. It can participate in many important biochemical reactions in the body, protect the important enzymes in the body from oxidative and inactivation, and ensure energy metabolism and cell utilization. At the same time, through the combination of thiol and free radicals in the body, it can directly reduce the free radicals into acidic substances, thereby accelerating the excretion of free radicals and countering the damage of free radicals to important organs. Haddad et al found that GSH is involved in lipopolysaccharide-induced regulation of cytokine transcription and regulation of I-KB/NF-KB signaling pathway. Armstrong et al found that the decrease in GSH content is a potential early activation signal of apoptosis, and the subsequent production of oxygen free radicals promotes cell apoptosis.
Glutathione (GSH) Probes
BOC Sciences offers a range of patented water-soluble and high light stability probes (dye molecules) for rapid detection and quantification of glutathione (GSH). Our probes can detect glutathione in absorption (colorimetry); absorbance (quantitative); fluorescence or fluorescence ratio (quantitative), without enzymes and long waiting times. Our probes (dye molecules) are very bright, easy to use, and provide highly repeatable results. GSH RedTM is a unique water-soluble, highly fluorescent probe that detects and quantifies glutathione (GSH). Its long absorption and emission wavelength make it ideal for flow cytometry, microscopy and HCS imaging.
Reference:
- Pompella A.; et al. The changing faces of glutathione, a cellular protagonist. Biochemical Pharmacology. 2003, 66 (8): 1499–503.
Resources

- Hoechst Dyes: Definition, Structure, Mechanism and Applications
- Mastering the Spectrum: A Comprehensive Guide to Cy3 and Cy5 Dyes
- Fluorescent Probes: Definition, Structure, Types and Application
- Fluorescent Dyes: Definition, Mechanism, Types and Application
- Coumarin Dyes: Definition, Structure, Benefits, Synthesis and Uses
- Unlocking the Power of Fluorescence Imaging: A Comprehensive Guide
- Cell Imaging: Definitions, Systems, Protocols, Dyes, and Applications
- Lipid Staining: Definition, Principles, Methods, Dyes, and Uses
- Flow Cytometry: Definition, Principles, Protocols, Dyes, and Uses
- Nucleic Acid Staining: Definition, Principles, Dyes, Procedures, and Uses
Online Inquiry

